计算准简单多组分水-电解质体系溶解度图的算法(程序变体
IF 2.1
3区 工程技术
Q3 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
本文致力于研究准简单体系的等温-等压溶解度图。准简单体系是多组分体系,在这些体系中,溶剂活性恒定线(面、超曲面)的特征是液体溶液组成变量的线性相互依存关系。在这种体系中,不完全吉布斯势对组成变量的具体依赖类型是确定的。介绍了计算这些体系的等温线-等压溶解度图的通用算法,该算法与电解质的价型、体系的组分数量和固相类型无关,也可扩展到已形成固溶体的体系。溶解度图的非模型热力学计算结果与文献中的实验数据一致,令人信服。本文章由计算机程序翻译,如有差异,请以英文原文为准。
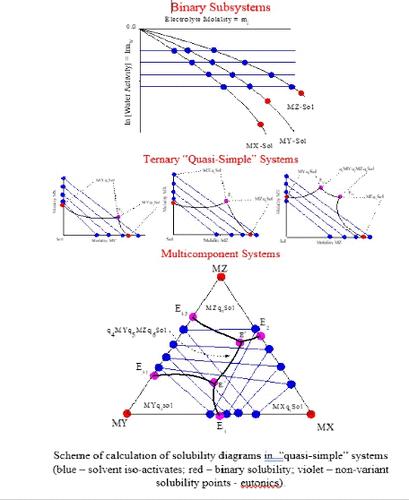
Algorithm (Procedure Variants) for the Calculation of Solubility Diagrams of Quasi-Simple Multicomponent Water–Electrolyte Systems
This paper is devoted to the consideration of isotherm–isobaric solubility diagrams of quasi-simple systems. Quasi-simple systems are multicomponent systems in which the lines (surfaces, hypersurfaces) of constancy solvent activity are characterized by a linear interdependence of the variables of the composition of liquid solutions. The specific type of dependence of the incomplete Gibbs potential on the composition variables for such systems is determined. A universal algorithm for the calculation of isotherm-isobaric solubility diagrams of these systems, independent of the valence type of the electrolyte, the number of components of the system, and the types of solid phases, which is also extended to systems with solid solutions formed, is presented. The results of nonmodel thermodynamic calculations of solubility diagrams are in convincing agreement with experimental data available in the literature.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Chemical & Engineering Data
工程技术-工程:化工
CiteScore
5.20
自引率
19.20%
发文量
324
审稿时长
2.2 months
期刊介绍:
The Journal of Chemical & Engineering Data is a monthly journal devoted to the publication of data obtained from both experiment and computation, which are viewed as complementary. It is the only American Chemical Society journal primarily concerned with articles containing data on the phase behavior and the physical, thermodynamic, and transport properties of well-defined materials, including complex mixtures of known compositions. While environmental and biological samples are of interest, their compositions must be known and reproducible. As a result, adsorption on natural product materials does not generally fit within the scope of Journal of Chemical & Engineering Data.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


