293.15 至 333.15 K 温度下 4-乙酰氧基氮杂环丁-2-酮在 12 种纯溶剂和一种二元溶剂中的溶解度测量与模型相关性
IF 2
3区 工程技术
Q3 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
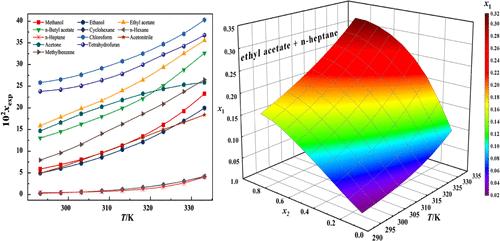
在 293.15 至 333.15 K 的温度范围内,采用动态激光监测法测定了 4-乙酰氧基氮杂环丁烷-2-酮(4-AA)在 12 种纯溶剂(甲醇、乙醇、乙酸乙酯、乙酸正丁酯、环己烷、正己烷、正庚烷、四氢呋喃、氯仿、乙腈、丙酮和甲苯)和一种二元溶剂体系(乙酸乙酯 + 正庚烷)中的溶解度。结果表明,4-AA 的溶解度随着温度的升高和二元溶剂中乙酸乙酯摩尔分数的增加而增加。使用了八种模型(Apelblat、Van't Hoff、λh、Wilson、NRTL、CNIBS/R-K、Apelblat-Jouyban-Acree 和 Van't Hoff-Jouyban-Acree)来关联溶解度数据。结果表明,在纯溶剂中,Van't Hoff 模型与实验值的相关性最好,而在二元溶剂体系中,CNIBS/R-K 模型的拟合精度最高。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Solubility Measurement and Model Correlation of 4-Acetoxyazetidin-2-one in 12 Pure Solvents and One Binary Solvent at 293.15 to 333.15 K
The solubility of 4-acetoxyazetidin-2-one (4-AA) in twelve pure solvents (methanol, ethanol, ethyl acetate, n-butyl acetate, cyclohexane, n-hexane, n-heptane, tetrahydrofuran, chloroform, acetonitrile, acetone, and toluene) and one binary solvent system (ethyl acetate + n-heptane) was measured by a dynamic laser monitoring method at temperatures ranging from 293.15 to 333.15 K. The results indicated that the solubility of 4-AA increased with rising temperature and with a higher molar fraction of ethyl acetate in the binary solvent. Eight models (Apelblat, Van’t Hoff, λh, Wilson, NRTL, CNIBS/R–K, Apelblat–Jouyban–Acree, and Van’t Hoff–Jouyban–Acree) were used to correlate the solubility data. The results showed that the Van’t Hoff model best correlated with the experimental values in pure solvent, while, the CNIBS/R–K model demonstrated the highest fitting accuracy in the binary solvent system.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Chemical & Engineering Data
工程技术-工程:化工
CiteScore
5.20
自引率
19.20%
发文量
324
审稿时长
2.2 months
期刊介绍:
The Journal of Chemical & Engineering Data is a monthly journal devoted to the publication of data obtained from both experiment and computation, which are viewed as complementary. It is the only American Chemical Society journal primarily concerned with articles containing data on the phase behavior and the physical, thermodynamic, and transport properties of well-defined materials, including complex mixtures of known compositions. While environmental and biological samples are of interest, their compositions must be known and reproducible. As a result, adsorption on natural product materials does not generally fit within the scope of Journal of Chemical & Engineering Data.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


