用于细胞内给药和骨再生的抗衰老代谢产物基聚合物微粒
IF 11.1
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
摘要
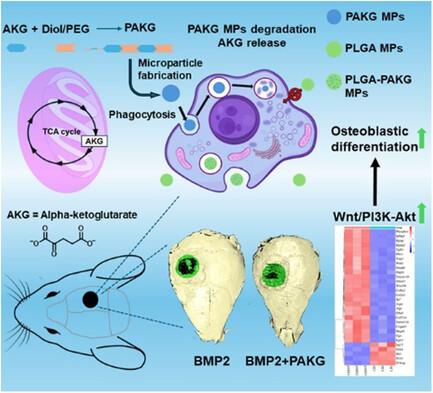
α-酮戊二酸(AKG)是三羧酸循环的一种关键成分,因其抗衰老特性而备受关注。最近的研究表明,局部给药的细胞渗透性 AKG 能显著促进成骨分化和小鼠骨再生。然而,该代谢产物的细胞毒性和快速水解限制了其应用。本研究合成了新型的基于 AKG 的聚合物微颗粒(PAKG MPs),用于持续释放。体外数据表明,微颗粒的化学成分、亲水性和大小会显著影响其细胞毒性和促骨质生成活性。令人兴奋的是,这些可生物降解的 PAKG MPs 对非吞噬性前成骨细胞 MC3T3-E1 和原发性骨髓间充质干细胞具有很强的吞噬能力,能显著促进它们的成骨分化。RNA测序(RNA-Seq)数据表明,PAKG MPs能强烈激活Wnt/β-catenin和PI3K-Akt通路,促进成骨分化。此外,PAKG 还能使聚(L-乳酸)和聚(乳酸-共聚乙醇酸)MPs(PLGA MPs)高效吞噬。这些数据表明,与PLGA-MPs递送的非那米相比,PLGA-PAKG-MPs介导的细胞内药物递送能显著促进成骨细胞的分化。值得注意的是,PAKG MPs 能显著改善小鼠颅骨缺损模型中的大骨再生。因此,基于 PAKG 的新型 MPs 在改善成骨细胞分化和骨再生方面大有可为,并能为广泛的再生医学提供高效的细胞内给药。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Antiaging Metabolite-Based Polymeric Microparticles for Intracellular Drug Delivery and Bone Regeneration
α-ketoglutarate (AKG), a key component of the tricarboxylic acid cycle, has attracted attention for its antiaging properties. In the recent study, it is indicated that locally delivered cell-permeable AKG significantly promotes osteogenic differentiation and mouse bone regeneration. However, the cytotoxicity and rapid hydrolysis of the metabolite limit its application. In this study, novel AKG-based polymeric microparticles (PAKG MPs) are synthesized for sustained release. In vitro data suggest that the chemical components, hydrophilicity, and size of the MPs can significantly affect their cytotoxicity and pro-osteogenic activity. Excitingly, these biodegradable PAKG MPs are highly phagocytosable for nonphagocytic pre-osteoblasts MC3T3-E1 and primary bone marrow mesenchymal stem cells, significantly promoting their osteoblastic differentiation. RNA-Sequencing (RNA-Seq) data suggest that PAKG MPs strongly activate Wnt/β-catenin and PI3K–Akt pathways for osteogenic differentiation. Moreover, PAKG enables poly(L-lactic acid) and poly(lactic-co-glycolic acid) MPs (PLGA MPs) for efficient phagocytosis. In this data, it is indicated that PLGA–PAKG-MPs-mediated intracellular drug delivery can significantly promote stronger osteoblastic differentiation compared to PLGA-MPs-delivered phenamil. Notably, PAKG MPs significantly improve large bone regeneration in a mouse cranial bone defect model. Thus, the novel PAKG-based MPs show great promise to improve osteogenic differentiation and bone regeneration and enable efficient intracellular drug delivery for broad regenerative medicine.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
14.00
自引率
2.40%
发文量
0
期刊介绍:
Small Science is a premium multidisciplinary open access journal dedicated to publishing impactful research from all areas of nanoscience and nanotechnology. It features interdisciplinary original research and focused review articles on relevant topics. The journal covers design, characterization, mechanism, technology, and application of micro-/nanoscale structures and systems in various fields including physics, chemistry, materials science, engineering, environmental science, life science, biology, and medicine. It welcomes innovative interdisciplinary research and its readership includes professionals from academia and industry in fields such as chemistry, physics, materials science, biology, engineering, and environmental and analytical science. Small Science is indexed and abstracted in CAS, DOAJ, Clarivate Analytics, ProQuest Central, Publicly Available Content Database, Science Database, SCOPUS, and Web of Science.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


