用于癌症免疫疗法的 STING 激活聚合物-药物共轭物
IF 10.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
干扰素基因刺激器(STING)通路连接着先天性和适应性抗肿瘤免疫,因此在癌症免疫监视中发挥着重要作用。这促使用于癌症免疫疗法的 STING 激动剂得到广泛开发,但药理学障碍继续限制着 STING 激动剂的临床影响,并促使人们开发药物输送系统以提高其疗效和/或安全性。我们开发了一种 STING 激活聚合物-药物共轭物平台 SAPCon,该平台基于应变促进叠氮-炔烃环加成法,将新型二聚氨基苯并咪唑(diABZI)STING 原药与亲水性聚(二甲基丙烯酰胺-叠氮-乙基甲基丙烯酸酯)聚合物链通过 cathepsin B 响应连接体连接,以延长循环时间并实现被动肿瘤蓄积。我们发现,静脉注射的 SAPCon 会在肿瘤部位积聚,并被肿瘤相关的髓系细胞内吞,导致肿瘤组织中 STING 激活增加。因此,SAPCon 促进了以活化巨噬细胞和树突状细胞频率增加以及 CD8+ T 细胞浸润改善为特征的免疫原性肿瘤微环境,从而抑制了肿瘤生长,延长了生存期,并增强了正位乳腺癌模型对抗 PD-1 免疫检查点阻断的反应。总之,这些研究将 SAPCon 定位为一个模块化、可编程的平台,用于提高全身给药 STING 激动剂在癌症免疫疗法中的疗效。本文章由计算机程序翻译,如有差异,请以英文原文为准。
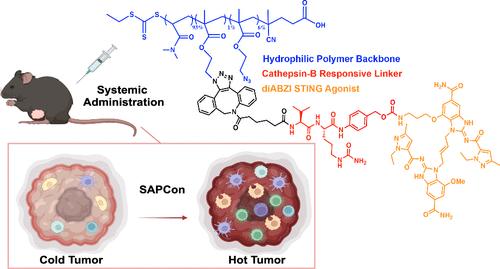
STING-Activating Polymer–Drug Conjugates for Cancer Immunotherapy
The stimulator of interferon genes (STING) pathway links innate and adaptive antitumor immunity and therefore plays an important role in cancer immune surveillance. This has prompted widespread development of STING agonists for cancer immunotherapy, but pharmacological barriers continue to limit the clinical impact of STING agonists and motivate the development of drug delivery systems to improve their efficacy and/or safety. We developed SAPCon, a STING-activating polymer–drug conjugate platform based on strain-promoted azide–alkyne cycloaddition of a novel dimeric amidobenzimidazole (diABZI) STING prodrug to hydrophilic poly(dimethylacrylamide-co-azido-ethylmethacrylate) polymer chains through a cathepsin B-responsive linker to increase circulation time and enable passive tumor accumulation. We found that intravenously administered SAPCon accumulated at tumor sites, where it was endocytosed by tumor-associated myeloid cells, resulting in increased STING activation in the tumor tissue. Consequently, SAPCon promoted an immunogenic tumor microenvironment characterized by increased frequency of activated macrophages and dendritic cells and improved infiltration of CD8+ T cells, resulting in inhibition of tumor growth, prolonged survival, and enhanced response to anti-PD-1 immune checkpoint blockade in orthotopic breast cancer models. Collectively, these studies position SAPCon as a modular and programmable platform for improving the efficacy of systemically administered STING agonists for cancer immunotherapy.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Central Science
Chemical Engineering-General Chemical Engineering
CiteScore
25.50
自引率
0.50%
发文量
194
审稿时长
10 weeks
期刊介绍:
ACS Central Science publishes significant primary reports on research in chemistry and allied fields where chemical approaches are pivotal. As the first fully open-access journal by the American Chemical Society, it covers compelling and important contributions to the broad chemistry and scientific community. "Central science," a term popularized nearly 40 years ago, emphasizes chemistry's central role in connecting physical and life sciences, and fundamental sciences with applied disciplines like medicine and engineering. The journal focuses on exceptional quality articles, addressing advances in fundamental chemistry and interdisciplinary research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


