n→π* 相互作用的保利排斥:对古生物学的影响
IF 12.7
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
蛋白质的进化是为了在水环境中发挥作用。为动物提供身体支架的胶原蛋白特别需要保持其完整性。尽管肽键在中性水溶液中的半衰期只有 500 年,但在恐龙化石中发现了完整的胶原蛋白。我们试图发现这种显著的抗水解性的物理化学基础。利用实验和计算方法,我们发现主链酰基可以通过与邻近酰基的 O-C═O n→π* 相互作用来防止水解。这些相互作用几乎涉及胶原蛋白三螺旋中的每一个肽键。这种源于保利排斥原理的保护作用可能是古老胶原蛋白得以保存的原因。本文章由计算机程序翻译,如有差异,请以英文原文为准。
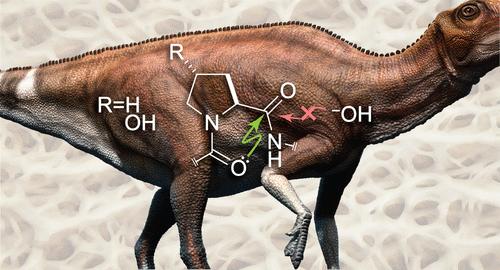
Pauli Exclusion by n→π* Interactions: Implications for Paleobiology
Proteins have evolved to function in an aqueous environment. Collagen, which provides the bodily scaffold for animals, has a special need to retain its integrity. This need was addressed early on, as intact collagen has been detected in dinosaur fossils, even though peptide bonds have a half-life of only ∼500 years in a neutral aqueous solution. We sought to discover the physicochemical basis for this remarkable resistance to hydrolysis. Using experimental and computational methods, we found that a main-chain acyl group can be protected from hydrolysis by an O···C═O n→π* interaction with a neighboring acyl group. These interactions engage virtually every peptide bond in a collagen triple helix. This protection, which arises from the Pauli exclusion principle, could underlie the preservation of ancient collagen.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Central Science
Chemical Engineering-General Chemical Engineering
CiteScore
25.50
自引率
0.50%
发文量
194
审稿时长
10 weeks
期刊介绍:
ACS Central Science publishes significant primary reports on research in chemistry and allied fields where chemical approaches are pivotal. As the first fully open-access journal by the American Chemical Society, it covers compelling and important contributions to the broad chemistry and scientific community. "Central science," a term popularized nearly 40 years ago, emphasizes chemistry's central role in connecting physical and life sciences, and fundamental sciences with applied disciplines like medicine and engineering. The journal focuses on exceptional quality articles, addressing advances in fundamental chemistry and interdisciplinary research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


