量身定制的吡唑-苯并噻唑混合物的合成、抗癌评估、硅内 ADMET 和分子对接研究
IF 2.8
4区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
本文展示了 18 种新型吡唑-苯并噻唑杂化分子 5a-5r 的区域选择性合成、表征和生物学评价。我们利用 β,β-二丁氧基酮协议合成了这些杂化分子。我们使用 MTT 法测试了合成的化合物对乳腺癌(MCF-7)、宫颈癌(HeLa)和肺癌(A549)细胞株的体外抗增殖活性。混合分子 5a、5m、5n 和 5o 的 IC50 值分别为 0.359 mM、0.051 mM、0.079 mM 和 0.259 mM,它们对 MCF-7 癌细胞的生长抑制活性令人赞叹,甚至优于 IC50 值为 0.439 mM 的卡铂参考药物。化合物 5k 的 IC50 值为 0.765 mM,是对 HeLa 癌细胞最有效的抗增殖剂。此外,与 IC50 值为 0.805 mM 的卡铂参考药物相比,IC50 值为 0.706 mM 的杂化分子 5f 对 A549 癌细胞具有更好的抑制活性。研究人员利用Annexin V-FITC/PI双染色法和细胞周期测定法对细胞毒性机制进行了研究。在 VEGFR-2 位点的结合口袋(PBD 代码:4ASD)中对所有合成化合物进行了分子对接研究。最后,还研究了强效分子的 ADMET 特征,以预测其药物相似性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
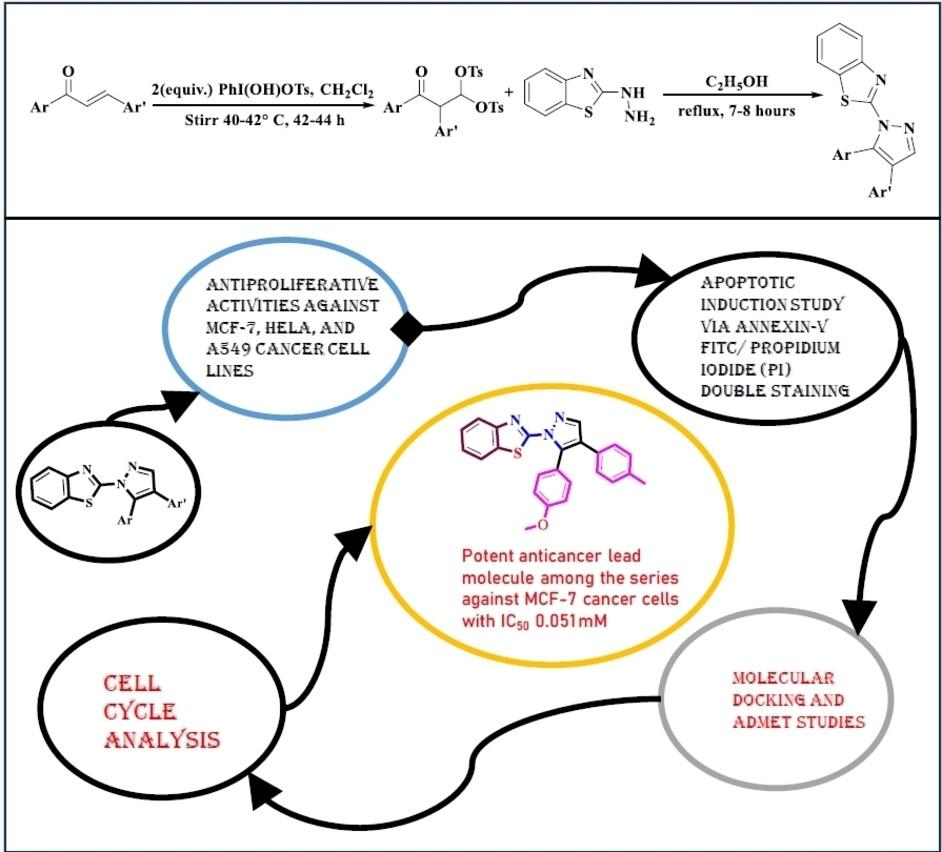
Synthesis, Anticancer Evaluation, in‐silico ADMET and Molecular Docking Studies for Tailored Pyrazolo‐Benzothiazole Hybrids
The present article demonstrates the regioselective synthesis, characterization, and biological evaluation of eighteen novel pyrazolo‐benzothiazole hybrid molecules 5a–5r. We have utilized β,β‐ditosyloxy ketone protocol to synthesize these hybrid molecules. The synthesized compounds were tested for their in‐vitro antiproliferative activities using MTT assay against breast cancer (MCF‐7), cervical cancer (HeLa), and Lung cancer (A549) cell lines. Hybrid molecules 5a, 5m, 5n, and 5o with IC50 values of 0.359 mM, 0.051 mM, 0.079 mM, and 0.259 mM respectively exhibited admirable growth inhibitory properties against MCF‐7 cancer cells which are even better than reference carboplatin drug having IC50 (0.439 mM). Compound 5k with IC50 value of 0.765 mM was found to be the most potent antiproliferative agent for the HeLa cancer cells. Moreover, hybrid molecule 5f with IC50 value of 0.706 mM exhibited better inhibitory activity against A549 cancer cells in comparison to the reference carboplatin drug having IC50 (0.805 mM). The mechanism of cellular toxicity was studied using the Annexin V‐FITC/PI double staining method and cell cycle assay. Molecular docking studies for all the synthesized compounds have also been performed in the binding pocket of VEGFR2 sites (PDB code: 4ASD). Finally, the ADMET profile of the potent molecules was investigated to predict their drug‐likeness behaviour.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Asian Journal of Organic Chemistry
CHEMISTRY, ORGANIC-
CiteScore
4.70
自引率
3.70%
发文量
372
期刊介绍:
Organic chemistry is the fundamental science that stands at the heart of chemistry, biology, and materials science. Research in these areas is vigorous and truly international, with three major regions making almost equal contributions: America, Europe and Asia. Asia now has its own top international organic chemistry journal—the Asian Journal of Organic Chemistry (AsianJOC)
The AsianJOC is designed to be a top-ranked international research journal and publishes primary research as well as critical secondary information from authors across the world. The journal covers organic chemistry in its entirety. Authors and readers come from academia, the chemical industry, and government laboratories.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


