FimH 拮抗剂及其类似物的简易合成:大肠杆菌粘附的复杂 C-甘露糖苷抑制剂的简单入口
IF 4
3区 医学
Q2 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
由于 FimH 拮抗剂的 C-甘露糖苷结构具有密集的官能度和复杂的立体化学结构,因此合成 FimH 拮抗剂具有挑战性,导致产量低、过程长。我们提出了一种通过镍催化还原偶联和立体控制还原合成 C-甘露糖苷 FimH 拮抗剂的高效方法,从而大大简化了合成过程,只需四个步骤就能合成 FimH 拮抗剂,总产率高达 34-50%。这种高效的合成方法为快速开发治疗由大肠杆菌(E. coli)引起的尿路感染或克罗恩病的类似物提供了巨大的潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
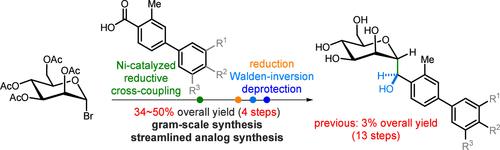
Facile Synthesis of FimH Antagonist and Its Analogues: Simple Entry to Complex C-Mannoside Inhibitors of E. coli Adhesion
Synthesizing FimH antagonists is challenging because of their densely functionalized and stereochemically complex C-mannoside structures, resulting in low yields and lengthy processes. We present an efficient method for synthesizing C-mannoside FimH antagonists by nickel-catalyzed reductive coupling and stereocontrolled reduction, thereby significantly simplifying the process and enabling the synthesis of FimH antagonists in just four steps with an overall yield of 34–50%. This efficient synthesis holds significant potential for the rapid development of analogues targeting the treatment of urinary tract infections or Crohn’s disease caused by Escherichia coli (E. coli).
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
ACS Medicinal Chemistry Letters
CHEMISTRY, MEDICINAL-
CiteScore
7.30
自引率
2.40%
发文量
328
审稿时长
1 months
期刊介绍:
ACS Medicinal Chemistry Letters is interested in receiving manuscripts that discuss various aspects of medicinal chemistry. The journal will publish studies that pertain to a broad range of subject matter, including compound design and optimization, biological evaluation, drug delivery, imaging agents, and pharmacology of both small and large bioactive molecules. Specific areas include but are not limited to:
Identification, synthesis, and optimization of lead biologically active molecules and drugs (small molecules and biologics)
Biological characterization of new molecular entities in the context of drug discovery
Computational, cheminformatics, and structural studies for the identification or SAR analysis of bioactive molecules, ligands and their targets, etc.
Novel and improved methodologies, including radiation biochemistry, with broad application to medicinal chemistry
Discovery technologies for biologically active molecules from both synthetic and natural (plant and other) sources
Pharmacokinetic/pharmacodynamic studies that address mechanisms underlying drug disposition and response
Pharmacogenetic and pharmacogenomic studies used to enhance drug design and the translation of medicinal chemistry into the clinic
Mechanistic drug metabolism and regulation of metabolic enzyme gene expression
Chemistry patents relevant to the medicinal chemistry field.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


