强效、选择性和口服生物活性分子胶降解剂 CK1α 的结构-活性关系
IF 4
3区 医学
Q2 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
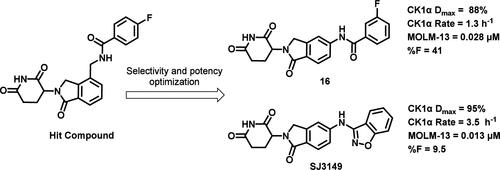
众所周知,最初的分子胶降解剂(沙利度胺、来那度胺和泊马度胺)可与脑龙(CRBN)结合并改变其表面,从而诱导具有治疗价值的新基质(IKZF1、IKZF3和CK1α)的招募、泛素化和降解。为了了解和调节新底物的特异性,我们最近报告发现了 SJ3149 (4),它是 CK1α 的一种选择性强效分子胶降解剂,在多种癌细胞系中具有活性。在此,我们介绍了发现 SJ3149 以及其他强效选择性 CK1α 降解剂的药物化学工作。我们报告了 CK1α 降解的动力学剖析和参数、三元复合物、抗增殖作用、体外 ADME 数据以及体内药代动力学研究,并证明了其口服生物利用度。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Structure–Activity Relationship of Potent, Selective, and Orally Bioavailable Molecular Glue Degraders of CK1α
The original molecular glue degraders (thalidomide, lenalidomide, and pomalidomide) are known to bind to cereblon (CRBN) and alter its surface to induce recruitment, ubiquitination, and degradation of therapeutically valuable neosubstrates (IKZF1, IKZF3, and CK1α). With the aim of understanding and modulating neosubstrate specificity, we recently reported the discovery of SJ3149 (4), a selective and potent molecular glue degrader of CK1α, that is active in multiple cancer cell lines. Herein, we describe the medicinal chemistry efforts that resulted in the discovery of SJ3149 as well as other potent and selective CK1α degraders. We report kinetic profiling and parameters of CK1α degradation, ternary complex, antiproliferative effects, in vitro ADME data, and in vivo pharmacokinetic studies with demonstrated oral bioavailability.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
ACS Medicinal Chemistry Letters
CHEMISTRY, MEDICINAL-
CiteScore
7.30
自引率
2.40%
发文量
328
审稿时长
1 months
期刊介绍:
ACS Medicinal Chemistry Letters is interested in receiving manuscripts that discuss various aspects of medicinal chemistry. The journal will publish studies that pertain to a broad range of subject matter, including compound design and optimization, biological evaluation, drug delivery, imaging agents, and pharmacology of both small and large bioactive molecules. Specific areas include but are not limited to:
Identification, synthesis, and optimization of lead biologically active molecules and drugs (small molecules and biologics)
Biological characterization of new molecular entities in the context of drug discovery
Computational, cheminformatics, and structural studies for the identification or SAR analysis of bioactive molecules, ligands and their targets, etc.
Novel and improved methodologies, including radiation biochemistry, with broad application to medicinal chemistry
Discovery technologies for biologically active molecules from both synthetic and natural (plant and other) sources
Pharmacokinetic/pharmacodynamic studies that address mechanisms underlying drug disposition and response
Pharmacogenetic and pharmacogenomic studies used to enhance drug design and the translation of medicinal chemistry into the clinic
Mechanistic drug metabolism and regulation of metabolic enzyme gene expression
Chemistry patents relevant to the medicinal chemistry field.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


