优化 MOF 中孔隙的微环境,提高三元组分混合物中乙烯的纯化率
IF 11.3
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
设计一种能同时吸附乙炔(C2H2)和乙烷(C2H6)杂质的吸附剂以一步法提纯乙烯(C2H4)仍然是一项挑战。在此,我们构建了一种新型铜基金属有机框架 (MOF)--BUT-321,它可以选择性地吸附 C2H2 和 C2H6,而不吸附 C2H4。研究发现,与等结构 MOF BUT-320 相比,BUT-321 孔道内高密度的氧结合位点能为 C2H6 与 C2H2 的更强相互作用创造最佳环境。色谱柱突破实验证实了 BUT-321 从二元(C2H2/C2H4 或 C2H6/C2H4,1:1)和三元(C2H2/C2H4/C2H6,1:1:1)气体混合物中分离 C2H4 的优异性能。此外,BUT-321 在水和碱性溶液中具有良好的化学稳定性,同时还具有合成可扩展性、经济可行性和可回收性,因此有助于一步法 C2H4 纯化的应用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
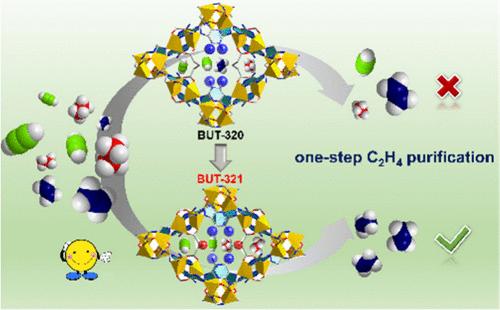
Optimizing the Microenvironment of Pores in an MOF for Boosting Ethylene Purification from a Ternary-Component Mixture
Designing an adsorbent that can simultaneously trap acetylene (C2H2) and ethane (C2H6) impurities for the one-step purification of ethylene (C2H4) remains a challenge. Herein, we constructed a novel Cu-based metal–organic framework (MOF), BUT-321, which exhibits the selective adsorption of C2H2 and C2H6 over C2H4. It was found that the high density of oxygen binding sites within the pore channels of BUT-321 can build an optimal environment for stronger interactions with C2H6, compared to the isostructural MOF BUT-320. Column breakthrough experiments confirm the exceptional C2H4 separation performance of BUT-321 from both binary (1:1 for C2H2/C2H4 or C2H6/C2H4) and ternary (1:1:1 for C2H2/C2H4/C2H6) gas mixtures in a single step. In addition, BUT-321 exhibits good chemical stability in water and an alkaline solution, combined with its synthesis scalability, economic viability, and recyclability, thus facilitating the application for the one-step C2H4 purification.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


