肿瘤治疗和疗效双模式自我评估成像的综合探针
IF 11.3
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
目前,独立的肿瘤治疗和评估方法无法实现实时评估。因此,迫切需要开发一种自我评估策略。据我们所知,目前还没有多模式自我评估策略。在此,我们设计了一种用于肿瘤治疗和双模态自我评估成像的综合探针(CPT-SS-DEVD-HCy)。在肿瘤细胞中,高浓度的谷胱甘肽(GSH)会特异性地诱导 CPT-SS-DEVD-HCy 释放 CPT,CPT-SS-DEVD-HCy 会激活 Caspase-3 (Casp-3),从而导致细胞凋亡。随后,活化的 Casp-3 进一步特异性地裂解多肽底物,释放出半氰基(HCy)染料,从而产生用于自我评估成像的荧光/光声(FL/PA)双模态信号。细胞实验表明,CPT-SS-DEVD-HCy 可通过 GSH 诱导的 CPT 释放有效杀死肿瘤细胞,并进一步成像凋亡过程中的 Casp-3 活性,FL/PA 信号增加了 5.1-/6.4 倍。此外,CPT-SS-DEVD-HCy 还显示出较高的肿瘤抑制效果,并能在体内对肿瘤治疗效果进行自我评估。我们希望这项工作能为将来开发更多的自我评估成像探针提供帮助。本文章由计算机程序翻译,如有差异,请以英文原文为准。
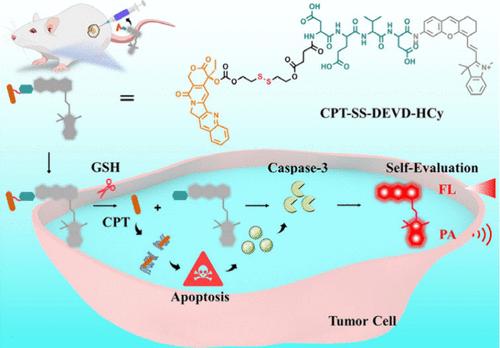
An Integrative Probe for Tumor Therapy and Dual-Modal Self-Evaluation Imaging of Therapeutic Efficacy
Currently, independent tumor therapy and evaluation methods can not achieve real-time evaluation. Therefore, development of a self-evaluation strategy is urgently needed. To the best of our knowledge, there is no multimodal self-evaluation strategy. Herein, we design an integrative probe (CPT-SS-DEVD-HCy) for tumor therapy and dual-modal self-evaluation imaging. In tumor cells, the high concentration of glutathione (GSH) specifically induces CPT-SS-DEVD-HCy to release CPT, which generates apoptosis with the activation of caspase-3 (Casp-3). Subsequently, activated Casp-3 further specifically cleaves peptide substrate to release hemicyanine (HCy) dye, thus generating a fluorescent/photoacoustic (FL/PA) dual-modal signal for self-evaluation imaging. Cell experiments show that CPT-SS-DEVD-HCy can effectively kill tumor cells through GSH-instructed CPT release and further image Casp-3 activity during apoptosis with a 5.1-/6.4-fold increased FL/PA signal. Moreover, CPT-SS-DEVD-HCy displays a high tumor inhibition effect and is competently able to self-evaluate the tumor therapeutic effect in vivo. We envision that this work will be helpful for developing more self-evaluation imaging probes in the future.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


