利用谷胱甘肽激活的光敏剂和近红外成像技术协同光动力疗法和化学动力疗法治疗肿瘤
IF 9.6
1区 化学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
摘要
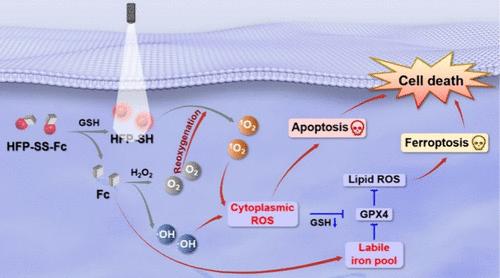
光动力疗法(PDT)利用光敏剂(PSs)在照射时产生细胞毒性活性氧(ROS),导致细胞死亡。然而,传统的光敏剂会产生不必要的副作用。可活化光敏剂(aPSs)提供了一种解决方案,它在肿瘤微环境中被触发前保持非活性。在这里,我们介绍一种新型谷胱甘肽(GSH)aPS--HFP-SS-Fc,它利用了肿瘤中较高的GSH浓度。HFP-SS-Fc 具有强效的近红外(NIR)荧光,暴露于肿瘤特异性的 GSH 水平时可产生强大的光动力效应。二硫键将二茂铁与 HFP-SS-Fc 连接起来,淬灭了近红外发射,阻碍了 ROS 的生成。这种二茂铁分子还能通过类似芬顿的反应促进-OH的产生和O2的释放,从而保持对缺氧肿瘤的疗效。受其体外治疗潜力的启发,HFP-SS-Fc 成功实现了实时肿瘤成像和显著的体内肿瘤生长抑制。这项研究表明,在近红外荧光的引导下,HFP-SS-Fc 是一种副作用最小的协同光动力疗法(PDT)和光动力疗法(CDT)的理想工具。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Synergistic Photodynamic and Chemodynamic Therapy for Tumor Treatment Using a Glutathione-Activated Photosensitizer with Near-Infrared (NIR) Imaging
Photodynamic therapy (PDT) utilizes photosensitizers (PSs) to generate cytotoxic reactive oxygen species (ROS) upon irradiation, leading to cell death. However, conventional PSs can cause unwanted side effects. Activatable photosensitizers (aPSs) offer a solution by remaining inactive until triggered within tumor microenvironment. Here, we present HFP-SS-Fc, a novel glutathione (GSH) aPS that leverages the high GSH concentration in tumors. HFP-SS-Fc exhibits potent near-infrared (NIR) fluorescence and a robust photodynamic effect upon exposure to tumor-specific GSH levels. A disulfide bond links ferrocene to HFP-SS-Fc, quenching NIR emission and hindering ROS generation. This ferrocene moiety also facilitates •OH production and O2 release through a Fenton-like reaction, maintaining efficacy in hypoxic tumors. Inspired by its theranostic potential in vitro, HFP-SS-Fc successfully achieves real-time tumor imaging and significant tumor growth inhibition in vivo. This study has presented HFP-SS-Fc as a promising tool for synergistic PDT and CDT with minimized side effects, guided by NIR fluorescence.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Materials Letters
MATERIALS SCIENCE, MULTIDISCIPLINARY-
CiteScore
14.60
自引率
3.50%
发文量
261
期刊介绍:
ACS Materials Letters is a journal that publishes high-quality and urgent papers at the forefront of fundamental and applied research in the field of materials science. It aims to bridge the gap between materials and other disciplines such as chemistry, engineering, and biology. The journal encourages multidisciplinary and innovative research that addresses global challenges. Papers submitted to ACS Materials Letters should clearly demonstrate the need for rapid disclosure of key results. The journal is interested in various areas including the design, synthesis, characterization, and evaluation of emerging materials, understanding the relationships between structure, property, and performance, as well as developing materials for applications in energy, environment, biomedical, electronics, and catalysis. The journal has a 2-year impact factor of 11.4 and is dedicated to publishing transformative materials research with fast processing times. The editors and staff of ACS Materials Letters actively participate in major scientific conferences and engage closely with readers and authors. The journal also maintains an active presence on social media to provide authors with greater visibility.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


