从 N-芳基羟胺中区域选择性合成 2-氨基苯酚
IF 2.7
3区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
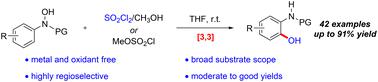
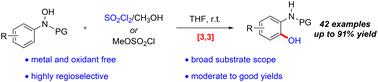
本研究描述了一种在无金属和氧化剂条件下合成 2-氨基苯酚的新策略。通过级联 [3,3]-sigmatropic 重排和原位水解过程,利用现成的 N-芳基羟胺和原位生成的氯磺酸甲酯,在温和的条件下从市售的硫酰氯和甲醇中高效地获得了 2-氨基苯酚。这种方法可以放大,底物范围广,对官能团有很好的耐受性和高区域选择性。在各种条件下,2-氨基苯酚产物都能以良好的收率转化为结构多样的功能分子。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Regioselective synthesis of 2-aminophenols from N-arylhydroxylamines†
A novel strategy for the synthesis of 2-aminophenols in the absence of metals and oxidants was described. The 2-aminophenols were efficiently obtained through a cascade [3,3]-sigmatropic rearrangement and in situ hydrolysis process by using readily available N-arylhydroxylamines and the in situ-generated methyl chlorosulfonate from commercially available sulfuryl chloride and methanol under mild conditions. This method could be scaled up and has a wide substrate scope with great functional group tolerance and high regioselectivity. The 2-aminophenol products could be readily converted into structurally diverse functional molecules in good yields under various conditions.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic & Biomolecular Chemistry
化学-有机化学
CiteScore
5.50
自引率
9.40%
发文量
1056
审稿时长
1.3 months
期刊介绍:
Organic & Biomolecular Chemistry is an international journal using integrated research in chemistry-organic chemistry. Founded in 2003 by the Royal Society of Chemistry, the journal is published in Semimonthly issues and has been indexed by SCIE, a leading international database. The journal focuses on the key research and cutting-edge progress in the field of chemistry-organic chemistry, publishes and reports the research results in this field in a timely manner, and is committed to becoming a window and platform for rapid academic exchanges among peers in this field. The journal's impact factor in 2023 is 2.9, and its CiteScore is 5.5.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


