二核和八核 1,2-二甲基吡啶鎓和 3-溴-1-乙基吡啶鎓碘锑酸盐(III):晶体结构和理化性质
IF 1.8
3区 化学
Q3 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
摘要
摘要 通过 SbI3 与相应阳离子的碘化物在有机溶剂中的反应,制备了新的锑配合物 (3-Br-1-EtPy)3[Sb2I9] (1) 和 (1,2-Me2Py)4[Sb8I28] (2)。化合物晶体结构的特征已通过 X 射线衍射测定。这些复合物的热稳定性至少可达 200°C,带隙约为 2.2 eV。本文章由计算机程序翻译,如有差异,请以英文原文为准。

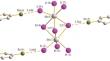
Di- and Octanuclear 1,2-Dimethylpyridinium and 3-Bromo-1-Ethylpyridinium Iodoantimonates(III): Crystal Structure and Physicochemical Properties
New antimony complexes, (3-Br-1-EtPy)3[Sb2I9] (1) and (1,2-Me2Py)4[Sb8I28] (2) have been prepared by the reaction of SbI3 with iodides of the corresponding cations in organic solvents. The features of the crystal structure of the compounds have been determined by X-ray diffraction. The complexes are thermally stable at least up to 200°C and have a band gap of ~2.2 eV.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Russian Journal of Inorganic Chemistry
化学-无机化学与核化学
CiteScore
3.10
自引率
38.10%
发文量
237
审稿时长
3 months
期刊介绍:
Russian Journal of Inorganic Chemistry is a monthly periodical that covers the following topics of research: the synthesis and properties of inorganic compounds, coordination compounds, physicochemical analysis of inorganic systems, theoretical inorganic chemistry, physical methods of investigation, chemistry of solutions, inorganic materials, and nanomaterials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


