探索氨与正十六烷在宽压力范围内的相互作用化学性质:实验和动力学模型研究
IF 5.3
3区 工程技术
Q2 ENERGY & FUELS
引用次数: 0
摘要
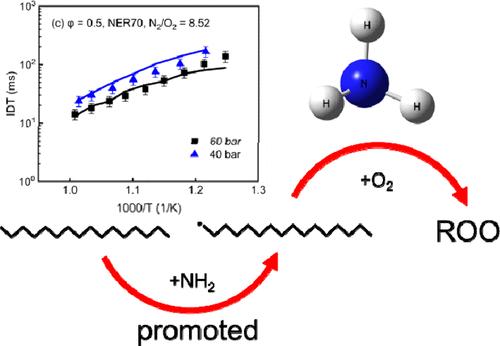
正十六烷(nC16H34)是表征柴油代用燃料中线性正构烷烃类的理想成分。在这项研究中,我们在快速压缩机中测量了不同 NH3 能量比(50%、70% 和 90%)、693-1047 K 温度、20-60 bar 压力和 0.5-1.0 等效比条件下 NH3/nC16H34 混合物的点火延迟时间 (IDT)。实验表明,在所研究的条件下,没有观察到明显的负温度系数(NTC)特征,IDT 随温度升高而单调下降。随着压力、N2/O2 比率和当量比的增加,IDT 缩短。对有重要更新的 nC16H34 和 NH3 子机制进行了合并,并在混合机制中增加了 C-N 交叉反应子集。建模结果表明,NH3/nC16H34 机制在大多数条件下都能很好地预测 IDT。敏感性分析表明,在化学计量条件下,IDTs 被高估的原因可能是反应 QOOH = 环醚 + OH 的速率规则存在误差,以及对高燃料和氧气含量条件下的动力学缺乏了解。NH3 和 nC16H34 之间的化学相互作用不容忽视,其中 NH2/RH 交叉反应子集在 IDT 建模中起着至关重要的作用。未来还需要进一步的量子计算来研究碳链长度对速率规则的影响,并获得对高燃料和氧气含量以及富氧条件下动力学的全面理解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Exploring the Interaction Chemistry of Ammonia with n-Hexadecane over Wide Pressure Ranges: An Experimental and Kinetic Modeling Study
Before applying ammonia (NH3)/hydrocarbon fuels in compression ignition engines, it is important to comprehensively understand the autoignition characteristics. n-Hexadecane (nC16H34) is an ideal component for characterizing the linear n-alkane class in diesel surrogates. In this investigation, the ignition delay times (IDTs) of NH3/nC16H34 mixtures were measured in a rapid compression machine at different NH3 energy ratios (50%, 70%, and 90%), temperatures of 693–1047 K, pressures of 20–60 bar, and equivalence ratios of 0.5–1.0. Experiments reveal that, under the investigated conditions, no distinguishable negative temperature coefficient (NTC) characteristic was observed, and the IDTs decrease monotonically with the increase of temperature. With the increase of pressure, N2/O2 ratio, and equivalence ratio, the IDTs are shortened. The nC16H34 and NH3 submechanisms with important updates were merged, and C-N cross-reaction subsets were added to the blending mechanism. Modeling results demonstrate that the NH3/nC16H34 mechanism predicts the IDTs well under most conditions. Sensitivity analysis shows that the overestimation of IDTs for stoichiometric conditions may be due to the errors of rate rules for reaction QOOH = cyclic ether + OH and a lack of kinetic understanding under the high fuel and oxygen content conditions. The interaction chemistry between NH3 and nC16H34 cannot be neglected, in which the NH2/RH cross-reaction subsets play a crucial role in modeling IDTs. Further quantum calculations are required to study the influence of carbon chain length on the rate rules and gain a comprehensive kinetic understanding of high fuel and oxygen contents and oxygen-rich conditions in the future.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Energy & Fuels
工程技术-工程:化工
CiteScore
9.20
自引率
13.20%
发文量
1101
审稿时长
2.1 months
期刊介绍:
Energy & Fuels publishes reports of research in the technical area defined by the intersection of the disciplines of chemistry and chemical engineering and the application domain of non-nuclear energy and fuels. This includes research directed at the formation of, exploration for, and production of fossil fuels and biomass; the properties and structure or molecular composition of both raw fuels and refined products; the chemistry involved in the processing and utilization of fuels; fuel cells and their applications; and the analytical and instrumental techniques used in investigations of the foregoing areas.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


