硝基苯的电化学加氢反应:从电催化到氧化还原介质催化
IF 7.2
2区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
引入聚氧化金属酸盐(POMs)作为氧化还原介质,可将直接电化学反应解耦为表面均相转化步骤。所形成的氧化还原催化作用有利于在低过电位条件下更有效地将硝基苯(Ph-NO2)氢化为苯胺(Ph-NH2)。值得注意的是,POM 的氧化还原电位可以调节氢化反应的能垒,从而提高转化效率。特别是使用磷钨酸({PW12})作为氧化还原介质,Ph-NO2 加氢为 Ph-NH2 的电位与 RHE 相比提高到 0.04 V,kapp 非常高,为 0.0339 min-1。这表明它的动力学性能优于之前报道的大多数电催化剂。此外,通过对电催化和氧化还原介质催化的机理比较研究,EC-SERS 揭示了介质分子的优先吸附及其与中间产物的直接相互作用。这项工作对于深入了解氧化还原介质的机理行为和潜在的调谐效应具有重要意义,有助于开发更高效的介质用于 Ph-NO2 的氢化。本文章由计算机程序翻译,如有差异,请以英文原文为准。
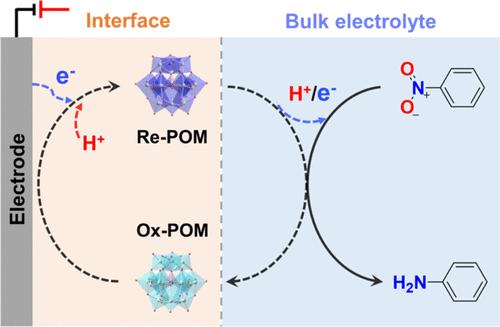
Electrochemical Hydrogenation of Nitrobenzene: From Electrocatalysis to Redox Mediator Catalysis
Introducing polyoxometalates (POMs) as redox mediators can decouple direct electrochemical reactions into surface-homogeneous conversion steps. And the formed redox catalysis is beneficial to achieve more efficient hydrogenation of nitrobenzene (Ph-NO2) to aniline (Ph-NH2) at low overpotentials. Notably, the redox potentials of POMs can adjust the energy barrier of the hydrogenation reaction, thus improving conversion efficiency. In particular, by using phosphotungstic acid ({PW12}) as the redox mediator, the potential of the hydrogenation of Ph-NO2 to Ph-NH2 was improved to 0.04 V vs RHE with a very high kapp of 0.0339 min–1. This indicates its superior kinetic performance over that of most previously reported electrocatalysts. In addition, through comparative mechanistic studies of electrocatalysis and redox mediator catalysis, EC-SERS revealed the preferential adsorption of mediator molecules and their direct interactions with intermediates. This work is significant to a deep understanding of the mechanistic behaviors and potential tuning effects of redox mediators, which will help to develop more efficient mediators for the hydrogenation of Ph-NO2.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemistry of Materials
工程技术-材料科学:综合
CiteScore
14.10
自引率
5.80%
发文量
929
审稿时长
1.5 months
期刊介绍:
The journal Chemistry of Materials focuses on publishing original research at the intersection of materials science and chemistry. The studies published in the journal involve chemistry as a prominent component and explore topics such as the design, synthesis, characterization, processing, understanding, and application of functional or potentially functional materials. The journal covers various areas of interest, including inorganic and organic solid-state chemistry, nanomaterials, biomaterials, thin films and polymers, and composite/hybrid materials. The journal particularly seeks papers that highlight the creation or development of innovative materials with novel optical, electrical, magnetic, catalytic, or mechanical properties. It is essential that manuscripts on these topics have a primary focus on the chemistry of materials and represent a significant advancement compared to prior research. Before external reviews are sought, submitted manuscripts undergo a review process by a minimum of two editors to ensure their appropriateness for the journal and the presence of sufficient evidence of a significant advance that will be of broad interest to the materials chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


