在高度稳定的双金属 Ni-Sn 合金催化剂上以糠醇为原料连续生产 1,2-戊二醇
IF 9.2
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
开发具有持久活性的长效催化剂对于将生物质持续转化为高附加值化合物至关重要。本研究展示了使用经济型双金属 Ni-Sn/ZnO 催化剂将糠醇 (FAL) 连续转化为 1,2-戊二醇 (1,2-PDO)。利用不同的镍/锰比例来表征催化表面并确定活性位点,揭示了镍锰合金相与相邻氧化锡之间的重要协同作用。这种协同作用大大增强了 FAL 和 THFA 中 δ-C5-O1 键的定向断裂,从而实现了 1,2-PDO的连续和选择性生产。镍和锡结合能的改变验证了高价锡电子转移对镍配位环境的改变。密度泛函理论研究结合实验结果表明,Ni3Sn2 (101) 平面通过 THFA 脱氢-裂解机制促进了 1,2-PDO 的选择性合成。此外,与 Ni3Sn 和金属镍相比,Ni3Sn2 相在吸附和离解 H2 方面表现出更强的能力。具有代表性的 3Ni-3Sn/ZnO 催化剂在将 FAL 连续转化为 1,2-PDO(收率为 91%)的过程中表现出很高的效率。值得注意的是,一个简单的再生过程就能恢复原有的催化活性,使 1,2-PDO 的连续生产时间达到 450 小时,共生产出 438 克(4464 毫摩尔)1,2-PDO。这一先进的催化系统展示了生物质转化的有效可扩展性,有利于环保的放大操作。本文章由计算机程序翻译,如有差异,请以英文原文为准。
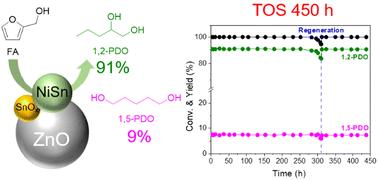
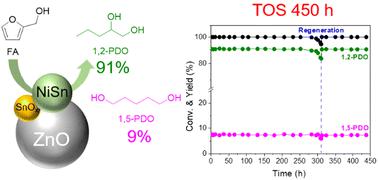
Continuous production of 1,2-pentanediol from furfuryl alcohol over highly stable bimetallic Ni–Sn alloy catalysts†
The development of robust long-lasting catalysts with enduring activity is essential for the sustainable conversion of biomass into value-added compounds. This study demonstrates the continuous conversion of furfuryl alcohol (FAL) into 1,2-pentanediol (1,2-PDO) using economical bimetallic Ni–Sn/ZnO catalysts. Various Ni/Sn ratios were used to characterize the catalytic surface and ascertain the active sites, revealing a vital collaborative impact between Ni–Sn alloy phases and adjacent SnOx species. This synergy significantly enhances the targeted breaking of the δ-C5–O1 bond in FAL and THFA, leading to continuous and selective 1,2-PDO production. The alteration in the binding energies of Ni and Sn validates the modification in the coordination environment of Ni caused by the transfer of electrons from highly valent Sn. Density functional theory studies combined with experimental results demonstrated that the Ni3Sn2 (101) plane promoted the selective synthesis of 1,2-PDO via the THFA dehydrogenation–scission mechanism. Furthermore, the Ni3Sn2 phase exhibits superior capability in adsorbing and dissociating H2 compared with Ni3Sn and metallic Ni. The representative 3Ni–3Sn/ZnO catalyst demonstrated high efficiency in the continuous conversion of FAL into 1,2-PDO with 91% yield, and a decline in conversion and selectivity was observed after 300 h because of the accumulation of carbonaceous species on the catalytic surface. Notably, a straightforward regeneration process restored the original catalytic activity, enabling the continuous production of 1,2-PDO for a total of ∼450 h, yielding 438 g (4464 mmol) of 1,2-PDO. This advanced catalytic system demonstrates effective scalability in biomass conversion, facilitating environmentally friendly scale-up operation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Green Chemistry
化学-化学综合
CiteScore
16.10
自引率
7.10%
发文量
677
审稿时长
1.4 months
期刊介绍:
Green Chemistry is a journal that provides a unique forum for the publication of innovative research on the development of alternative green and sustainable technologies. The scope of Green Chemistry is based on the definition proposed by Anastas and Warner (Green Chemistry: Theory and Practice, P T Anastas and J C Warner, Oxford University Press, Oxford, 1998), which defines green chemistry as the utilisation of a set of principles that reduces or eliminates the use or generation of hazardous substances in the design, manufacture and application of chemical products. Green Chemistry aims to reduce the environmental impact of the chemical enterprise by developing a technology base that is inherently non-toxic to living things and the environment. The journal welcomes submissions on all aspects of research relating to this endeavor and publishes original and significant cutting-edge research that is likely to be of wide general appeal. For a work to be published, it must present a significant advance in green chemistry, including a comparison with existing methods and a demonstration of advantages over those methods.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


