可回收皮啶酰胺衍生配体控制的炔烃与醇和酚的支链选择性酯化反应
IF 9.2
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
炔烃的氢酯化反应是一种重要的合成转化过程,可直接生成具有高原子经济性的酯。最近的进展主要集中在提高反应的选择性、效率和环境可持续性上,特别是通过配体和催化剂的创新,使该工艺在工业应用中更加实用。在此,我们报告了一种使用新型可回收配体对炔烃进行高选择性和高效氢酯化的方法,该配体可用于多种炔烃底物以及各种醇和酚。反应在温和的条件下进行,能以高产率和优异的区域选择性获得所需的酯。这种方法的一个显著特点是配体的可回收性,可以多次回收和重复使用,而不会明显丧失活性或选择性。机理研究表明,钯在纳米尺度上分散良好,对这种可持续的水解酯化过程至关重要。本文章由计算机程序翻译,如有差异,请以英文原文为准。
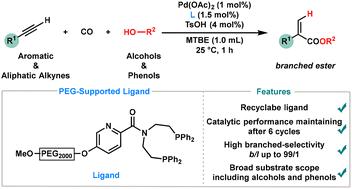
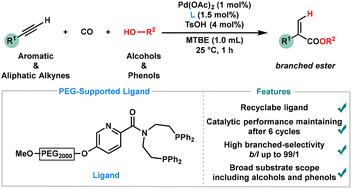
Recyclable picolinamide-derived ligand-controlled branched-selective hydroesterification of alkynes with alcohols and phenols†
Hydroesterification of alkynes is a crucial synthetic transformation, enabling the formation of esters directly with high atom economy. Recent advancements have centered on improving the reaction's selectivity, efficiency, and environmental sustainability, particularly through the innovation of ligands and catalysts, making the process more practical for industrial applications. Herein, we report a highly selective and efficient hydroesterification of alkynes using a novel recyclable ligand, accommodating a wide range of alkyne substrates as well as various alcohols and phenols. The reaction proceeds under mild conditions, affording the desired esters in high yields with excellent regioselectivities. A notable feature of this method is the recyclability of the ligand, which can be recovered and reused multiple times without significant loss of activity or selectivity. Mechanistic studies revealed that palladium was well dispersed on the nanoscale and was essential for this sustainable hydroesterification process.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Green Chemistry
化学-化学综合
CiteScore
16.10
自引率
7.10%
发文量
677
审稿时长
1.4 months
期刊介绍:
Green Chemistry is a journal that provides a unique forum for the publication of innovative research on the development of alternative green and sustainable technologies. The scope of Green Chemistry is based on the definition proposed by Anastas and Warner (Green Chemistry: Theory and Practice, P T Anastas and J C Warner, Oxford University Press, Oxford, 1998), which defines green chemistry as the utilisation of a set of principles that reduces or eliminates the use or generation of hazardous substances in the design, manufacture and application of chemical products. Green Chemistry aims to reduce the environmental impact of the chemical enterprise by developing a technology base that is inherently non-toxic to living things and the environment. The journal welcomes submissions on all aspects of research relating to this endeavor and publishes original and significant cutting-edge research that is likely to be of wide general appeal. For a work to be published, it must present a significant advance in green chemistry, including a comparison with existing methods and a demonstration of advantages over those methods.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


