在钽催化的氢氨基烷基化反应中利用天然复杂性实现底物控制的区域选择性和立体选择性
IF 9.3
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
天然存在且结构多样的含烯底物--萜烯--为在钽催化的氢氨基烷基化反应中建立化学、区域和非对映选择性反应提供了一个平台。天然衍生的 1,3-丁二烯揭示了通过氢氨基烷基化可从异戊二烯和 β-月桂烯获得的独特的区域和非对映选择性 (Z)-1,4 加成产物。在工业生产的松节油混合物中进行选择性萜烯官能化,证明了 β-蒎烯和柠檬烯的官能化特异性。最后,通过利用应变释放和立体电子效应来控制化学选择性,利用 β-石竹烯和葎草烯进行倍半萜官能化,为三取代烯烃在氢氨基烷基化中的反应性提供了罕见的实例。通过使用天然底物进行这些反应性研究,了解烯烃电子、应变和立体电子效应对化学选择性和非对映选择性结果影响的新工具揭示了氢氨基烷基化的新机理。本文章由计算机程序翻译,如有差异,请以英文原文为准。
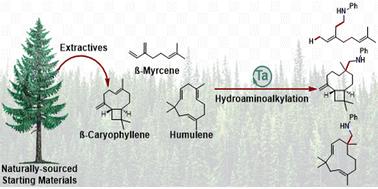
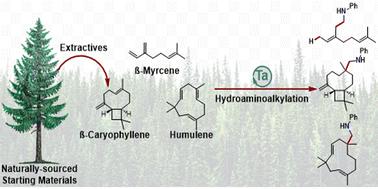
Exploiting natural complexity for substrate controlled regioselectivity and stereoselectivity in tantalum catalysed hydroaminoalkylation†
Naturally occurring and structurally diverse alkene-containing substrates, terpenes, provided a platform for establishing chemo-, regio-, and diastereoselective reactivity in tantalum catalysed hydroaminoalkylation. Naturally derived 1,3-butadienes revealed the unique regio- and diastereoselective (Z)-1,4-addition products accessible from isoprene and β-myrcene by hydroaminoalkylation. Selective terpene functionalisation, within an industrially produced turpentine mixture, demonstrates functionalisation specificity of β-pinene and limonene. Lastly, sesquiterpene functionalisation using β-caryophyllene and humulene provide rare examples of trisubstituted alkene reactivity in hydroaminoalkylation, by leveraging strain-release and stereoelectronic effects to control chemoselectivity. As a result of these reactivity studies using natural substrates, new tools for understanding alkene electronic, strain, and stereoelectronic effects on chemo- and diastereoselectivity outcomes have revealed new mechanistic insights into hydroaminoalkylation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Green Chemistry
化学-化学综合
CiteScore
16.10
自引率
7.10%
发文量
677
审稿时长
1.4 months
期刊介绍:
Green Chemistry is a journal that provides a unique forum for the publication of innovative research on the development of alternative green and sustainable technologies. The scope of Green Chemistry is based on the definition proposed by Anastas and Warner (Green Chemistry: Theory and Practice, P T Anastas and J C Warner, Oxford University Press, Oxford, 1998), which defines green chemistry as the utilisation of a set of principles that reduces or eliminates the use or generation of hazardous substances in the design, manufacture and application of chemical products. Green Chemistry aims to reduce the environmental impact of the chemical enterprise by developing a technology base that is inherently non-toxic to living things and the environment. The journal welcomes submissions on all aspects of research relating to this endeavor and publishes original and significant cutting-edge research that is likely to be of wide general appeal. For a work to be published, it must present a significant advance in green chemistry, including a comparison with existing methods and a demonstration of advantages over those methods.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


