利用芳基锍盐通过定点选择性 C-H 功能化实现通用电子供体-受体复合物介导的硫酯化反应
IF 9.2
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
合成硫代酯的现代方法通常需要昂贵的催化剂和苛刻的条件,因此从化学原料中合成硫代酯具有挑战性。在此,我们报告了一种可持续的金属、光催化剂和无氧化剂电子供体-受体(EDA)介导的硫代酯合成方法,该方法是在可见光照射下,使用芳基锍盐(受体)与硫酸钾盐(供体)进行位点选择性 C-H 功能化。我们的方法能以极高的效率和区域选择性从多种烯烃(包括医药和农用化合物)以及多种烷基、芳基和杂芳基硫代酸钾盐中快速获得硫代酯。机理研究支持 EDA 复合物的形成,而自由基捕获实验则证实了产物形成的自由基机理。此外,我们的方法还显示出极佳的原子经济性和 E 因子得分,在安全性、经济性和生态标准方面都堪称上乘。本文章由计算机程序翻译,如有差异,请以英文原文为准。
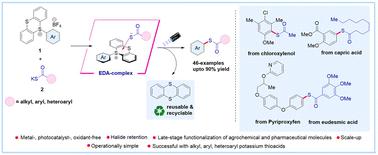
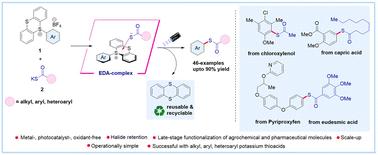
General electron–donor–acceptor complex mediated thioesterification reaction via site-selective C–H functionalization using aryl sulfonium Salts†
Contemporary methods for synthesizing thioesters often necessitate expensive catalysts and harsh conditions, making their synthesis from chemical feedstocks challenging. Herein, we report a sustainable metal-, photocatalyst-, and oxidant-free electron donor–acceptor (EDA) mediated synthesis of thioesters via site-selective C–H functionalization using aryl sulfonium salts (acceptor) with potassium thioacid salts (donor) under visible light irradiation. Our approach enables rapid access to thioesters from a wide variety of arenes, including pharmaceutical and agrochemical compounds, as well as a diverse range of alkyl, aryl, and heteroaryl potassium thioacid salts with excellent efficiency and regioselectivity. Mechanistic studies supported the formation of an EDA-complex, and radical trapping experiments corroborated the involvement of a radical-based mechanism for the product formation. Moreover, our method demonstrates excellent atom economy and E-factor scores, which are considered excellent in terms of safety, economic and ecological yardsticks.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Green Chemistry
化学-化学综合
CiteScore
16.10
自引率
7.10%
发文量
677
审稿时长
1.4 months
期刊介绍:
Green Chemistry is a journal that provides a unique forum for the publication of innovative research on the development of alternative green and sustainable technologies. The scope of Green Chemistry is based on the definition proposed by Anastas and Warner (Green Chemistry: Theory and Practice, P T Anastas and J C Warner, Oxford University Press, Oxford, 1998), which defines green chemistry as the utilisation of a set of principles that reduces or eliminates the use or generation of hazardous substances in the design, manufacture and application of chemical products. Green Chemistry aims to reduce the environmental impact of the chemical enterprise by developing a technology base that is inherently non-toxic to living things and the environment. The journal welcomes submissions on all aspects of research relating to this endeavor and publishes original and significant cutting-edge research that is likely to be of wide general appeal. For a work to be published, it must present a significant advance in green chemistry, including a comparison with existing methods and a demonstration of advantages over those methods.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


