环保型 Miyaura 硼酰化技术可实现绿色、1-锅硼酰化/铃木-Miyaura 偶联反应
IF 9.3
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
目前用于后续铃木-宫浦(SM)交叉偶联反应的硼酸及其相应酯类的制备路线会影响到与生成这些材料相关的成本、废物和安全问题。一种安装频哪醇乙硼酸酯或 B(Epin)衍生物的新方法,在相对较低的钯载量催化下,使用高浓度的绿色溶剂和适中的反应温度,在近乎无水的条件下生成稳定的硼酰化产物。另外,还可以在原位生成新的 Ar-B(表嘌呤),并直接在同一锅中用于 SM 反应,从而产生具有双芳基产物特征的芳香族和杂芳香族残留物。通过这种绿色环保的方法,可以生成一系列复杂的目标物,包括原料药相关产品。本文章由计算机程序翻译,如有差异,请以英文原文为准。
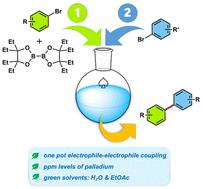
Environmentally friendly Miyaura Borylations allowing for green, 1-pot borylation/Suzuki–Miyaura couplings
Current routes to boronic acids and their corresponding esters to be used in subsequent Suzuki–Miyaura (SM) cross couplings impact the cost, waste, and safety concerns associated with generating these materials. A new method for installing the ethyl pinacol boronic ester, or B(Epin) derivative leads to stable borylated products under near-neat conditions using high concentrations of a green solvent and moderate reaction temperatures, catalyzed by relatively low palladium loadings. Alternatively, the newly fashioned Ar–B(Epin) can be generated in situ and used directly in the same pot for SM reactions leading to aromatic and heteroaromatic residues characteristic of the biaryl products being formed. An array of complex targets, including API-related products, can be generated via this green and environmentally responsible methodology.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Green Chemistry
化学-化学综合
CiteScore
16.10
自引率
7.10%
发文量
677
审稿时长
1.4 months
期刊介绍:
Green Chemistry is a journal that provides a unique forum for the publication of innovative research on the development of alternative green and sustainable technologies. The scope of Green Chemistry is based on the definition proposed by Anastas and Warner (Green Chemistry: Theory and Practice, P T Anastas and J C Warner, Oxford University Press, Oxford, 1998), which defines green chemistry as the utilisation of a set of principles that reduces or eliminates the use or generation of hazardous substances in the design, manufacture and application of chemical products. Green Chemistry aims to reduce the environmental impact of the chemical enterprise by developing a technology base that is inherently non-toxic to living things and the environment. The journal welcomes submissions on all aspects of research relating to this endeavor and publishes original and significant cutting-edge research that is likely to be of wide general appeal. For a work to be published, it must present a significant advance in green chemistry, including a comparison with existing methods and a demonstration of advantages over those methods.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


