掺杂微量 F 的 Co3O4 纳米针通过促进 OH 覆盖增强酸性水氧化活性
IF 9.2
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
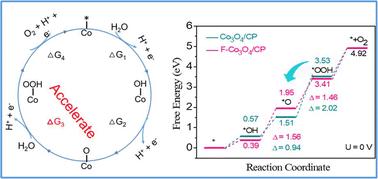
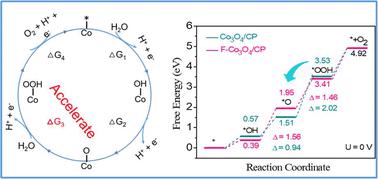
要降低清洁制氢的成本,就必须探索地球上丰富而高效的电催化剂,以取代酸性氧进化反应(OER)中的 Ir 和 Ru。在此,我们展示了微量电负性非金属元素氟(F)掺杂的 Co3O4 纳米针片可提高 Co3O4 的活性和稳定性。支撑在碳纸(F-Co3O4/CP)上的掺杂 F 的 Co3O4 纳米针在 10 mA cm-2 的酸性 OER 条件下显示出 350 mV 的过电位。详细研究表明,引入阴离子可促进 Co3O4 表面 OH 的富集,防止酸腐蚀,从而提高 OER 的内在活性和稳定性。理论计算进一步表明,掺杂 F 能有效改善电子传递,优化形成 *OOH 中间体的能垒,从而显著提高 OER 性能。这项研究为设计高效稳定的非贵金属酸性水氧化催化剂提供了指导。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Trace F-doped Co3O4 nanoneedles for enhanced acidic water oxidation activity via promoting OH coverage†
Exploring Earth-abundant and efficient electrocatalysts to replace Ir and Ru for the acidic oxygen evolution reaction (OER) is essential to reduce the cost of clean hydrogen production. Here, we show that trace amounts of electronegative non-metallic element fluorine (F)-doped Co3O4 nanoneedles improve the activity and stability of Co3O4. The F-doped Co3O4 nanoneedles supported on carbon paper (F-Co3O4/CP) exhibit an overpotential of 350 mV at 10 mA cm−2 for the acidic OER. In addition, their performance remains consistent after continuous operation for 80 h. Detailed investigations reveal that introducing an anion promotes the enrichment of OH on the surface of Co3O4 and prevents acid corrosion, thereby enhancing the intrinsic OER activity and stability. Theoretical calculations further indicate that F doping can effectively improve electron transfer and optimize the energy barrier for the formation of *OOH intermediates, which significantly improves OER performance. This study provides guidance for designing efficient and stable non-noble metal acidic water oxidation catalysts.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Green Chemistry
化学-化学综合
CiteScore
16.10
自引率
7.10%
发文量
677
审稿时长
1.4 months
期刊介绍:
Green Chemistry is a journal that provides a unique forum for the publication of innovative research on the development of alternative green and sustainable technologies. The scope of Green Chemistry is based on the definition proposed by Anastas and Warner (Green Chemistry: Theory and Practice, P T Anastas and J C Warner, Oxford University Press, Oxford, 1998), which defines green chemistry as the utilisation of a set of principles that reduces or eliminates the use or generation of hazardous substances in the design, manufacture and application of chemical products. Green Chemistry aims to reduce the environmental impact of the chemical enterprise by developing a technology base that is inherently non-toxic to living things and the environment. The journal welcomes submissions on all aspects of research relating to this endeavor and publishes original and significant cutting-edge research that is likely to be of wide general appeal. For a work to be published, it must present a significant advance in green chemistry, including a comparison with existing methods and a demonstration of advantages over those methods.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


