初级磺酰胺催化未活化烯烃的三组份羧化反应
IF 9.2
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
催化多组分反应为在单一步骤中构建多个化学键提供了一种高效的合成方法。然而,由于键能较高和步骤匹配方面的挑战,由 N-H 前体直接参与氮自由基的未活化烯烃羧化反应并不常见。在此,我们展示了一种使用简单伯氨基磺酸盐的催化自由基三组分反应,它为通过碳-氮和碳-碳键的形成构建复杂结构库提供了一种新方法。新开发的方法对各种官能团具有高度的耐受性,在非常温和的条件下高效地对复杂的药物分子和天然产物进行后期修饰。本文章由计算机程序翻译,如有差异,请以英文原文为准。
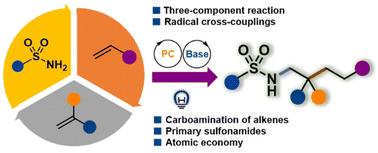
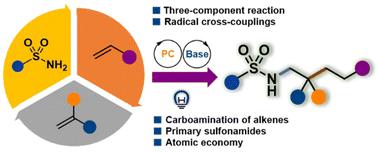
Catalytic three-component carboamination of unactivated alkenes with primary sulfonamides†
Catalytic multicomponent reactions offer an efficient synthetic approach for constructing multiple chemical bonds in a single step. However, the carboamination of unactivated alkenes, involving nitrogen radical species directly from N–H precursors, is infrequent due to the high bond energy and challenges in step matching. Herein we demonstrate a catalytic radical three-component reaction using simple primary sulfonamides, which provides a novel method for constructing a library of complex architectures through carbon–nitrogen and carbon–carbon bond formation. The newly developed method demonstrates a high degree of tolerance towards various functional groups, proving to be highly efficient in the late-stage modification of complex drug molecules and natural products under very mild conditions.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Green Chemistry
化学-化学综合
CiteScore
16.10
自引率
7.10%
发文量
677
审稿时长
1.4 months
期刊介绍:
Green Chemistry is a journal that provides a unique forum for the publication of innovative research on the development of alternative green and sustainable technologies. The scope of Green Chemistry is based on the definition proposed by Anastas and Warner (Green Chemistry: Theory and Practice, P T Anastas and J C Warner, Oxford University Press, Oxford, 1998), which defines green chemistry as the utilisation of a set of principles that reduces or eliminates the use or generation of hazardous substances in the design, manufacture and application of chemical products. Green Chemistry aims to reduce the environmental impact of the chemical enterprise by developing a technology base that is inherently non-toxic to living things and the environment. The journal welcomes submissions on all aspects of research relating to this endeavor and publishes original and significant cutting-edge research that is likely to be of wide general appeal. For a work to be published, it must present a significant advance in green chemistry, including a comparison with existing methods and a demonstration of advantages over those methods.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


