活化烯与二氧化碳和缺电子芳基溴的电化学羧化反应
IF 5.5
1区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
综合摘要在一个分子中同时构建两个邻接的 C-C 键仍然是一项重大挑战。在这项工作中,我们揭示了在温和、无过渡金属条件下活化烯烃的电还原羧化反应。利用现成的起始材料(缺电子芳基溴化物、活化烯和二氧化碳),该方法具有广泛的底物范围和良好的官能团耐受性。值得注意的是,这种方法能以高度化学和区域选择性的方式在烯烃上添加两种不同的亲电体。本文章由计算机程序翻译,如有差异,请以英文原文为准。
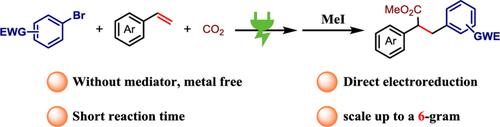
Electrochemical Carboarylation of Activated Alkenes with CO2 and Electron-deficient Aryl Bromides
The simultaneous construction of two vicinal C—C bonds in a molecule remains a significant challenge. In this work, we disclose an electroreductive carboarylation of activated alkenes under mild, transition metal-free conditions. Utilizing readily available starting materials (electron-deficient aryl bromides, activated alkenes, and CO2), this method demonstrates broad substrate scope and good functional group tolerance. Notably, this strategy enables the addition of two distinct electrophiles across an alkene in a highly chemo- and regioselective manner.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chinese Journal of Chemistry
化学-化学综合
CiteScore
8.80
自引率
14.80%
发文量
422
审稿时长
1.7 months
期刊介绍:
The Chinese Journal of Chemistry is an international forum for peer-reviewed original research results in all fields of chemistry. Founded in 1983 under the name Acta Chimica Sinica English Edition and renamed in 1990 as Chinese Journal of Chemistry, the journal publishes a stimulating mixture of Accounts, Full Papers, Notes and Communications in English.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


