通过 LiI/PPh3 催化光化学脱羧自由基烷基化/环化反应生成融合氮杂环
IF 5.5
1区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
综合摘要 开发了一种可见光诱导的 N-(2-氰基芳基)-丙烯酰胺与烷基 N-(酰氧基)邻苯二甲酰亚胺(NHPI 酯)之间的脱羧自由基级联环化反应,用于构建菲啶衍生物。这种方法利用碘化锂(LiI)和三苯基膦(PPh3)作为氧化还原催化剂,通过光激活电子供体-受体(EDA)复合物产生烷基自由基。在不使用过渡金属催化剂、化学氧化剂或基于金属/有机染料的光催化剂的情况下,制备了一系列伯、仲和叔烷基取代的菲啶类化合物,收率高达 82%。本文章由计算机程序翻译,如有差异,请以英文原文为准。
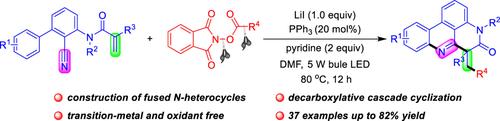
Photochemical Decarboxylative Radical Alkylation/Cyclization Reaction to Fused Nitrogen Heterocycles by LiI/PPh3 Catalysis
A visible-light-induced decarboxylative radical cascade cyclization reaction between N-(2-cyanoaryl)-acrylamides and alkyl N-(acyloxy)phthalimide (NHPI esters) for the construction of phenanthridine derivatives has been developed. This approach utilizes lithium iodide (LiI) and triphenylphosphine (PPh3) as the redox catalysts and the alkyl radical is produced through the photoactivation of the electron donor-acceptor (EDA) complex. A series of primary, secondary, and tertiary alkyl-substituted phenanthridines are prepared in up to 82% yield without transition-metal catalysts, chemical oxidants, or metal-/organic dye-based photocatalysts.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chinese Journal of Chemistry
化学-化学综合
CiteScore
8.80
自引率
14.80%
发文量
422
审稿时长
1.7 months
期刊介绍:
The Chinese Journal of Chemistry is an international forum for peer-reviewed original research results in all fields of chemistry. Founded in 1983 under the name Acta Chimica Sinica English Edition and renamed in 1990 as Chinese Journal of Chemistry, the journal publishes a stimulating mixture of Accounts, Full Papers, Notes and Communications in English.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


