二吩噻嗪衍生物异构化对光物理性质、晶体结构和力刺激响应的影响
IF 3.2
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
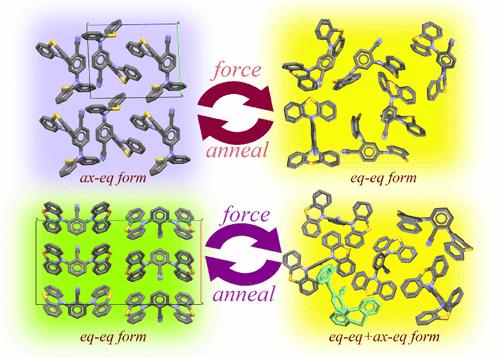
研究人员制备了两种 D-A-D 二吩噻嗪衍生物 24DPTCN 和 26DPTCN,以考察分子异构化对溶液和晶体状态下发光行为的影响以及对外力刺激的响应。研究发现,这两种化合物在溶液中有两条发射带。量子化学计算表明,两种构型的共存导致了两种发射带,短波长发射带来自 24DPTCN 和 26DPTCN 的轴-赤道(ax-eq)形式,而长波长发射带则归因于这些分子的赤道-赤道(eq-eq)形式。在结晶相中,24DPTCN 采用 ax-eq 形式,发出非常微弱的蓝色荧光,而 26DPTCN 则采用 eq-eq 构象,发出绿色荧光。更重要的是,它们对力刺激的反应也截然不同。在轻微的力刺激下,24DPTCN 的发射波段发生了 85 nm 的大幅红移,同时由于构象从 ax-eq 转换为 eq-eq 而导致发射强度增强。另一方面,在 26DPTCN 晶体被研磨后,由于少量等当量 26DPTCN 转移到了轴当量 26DPTCN 晶体,因此只观察到 28 nm 的转移,而且荧光减弱。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Impact of Isomerization of Diphenothiazine Derivatives on Photophysical Properties, Crystal Structure, and Force-Stimulus Response
Two D-A-D diphenothiazine derivatives, 24DPTCN and 26DPTCN, were prepared to investigate the impact of molecular isomerization on the luminescent behaviors in solution and crystalline states and the response to the external force stimuli. It was found that the two compounds had two emission bands in solutions. The quantum chemical calculations suggest that the coexistence of two configurations results in two emission bands, the short-wavelength bands are from the axial–equatorial (ax-eq) form for 24DPTCN and 26DPTCN, and the long-wavelength emission bands are ascribed to those molecules in equatorial–equatorial (eq-eq) form. In the crystalline phase, 24DPTCN adopts an ax-eq form and emits very weak blue fluorescence, and 26DPTCN has an eq-eq conformation and emits green fluorescence. More importantly, they also possess distinct response to force stimulus. 24DPTCN had a large redshift of 85 nm in the emission band under mild force stimulus, accompanied by an enhancement in the emission intensity because of the configuration conversion from ax-eq to eq-eq. On the other hand, only a shift of 28 nm was observed, and the fluorescence weakened after 26DPTCN crystals were ground because a small number of eq-eq 26DPTCN transferred into ax-eq ones.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Crystal Growth & Design
化学-材料科学:综合
CiteScore
6.30
自引率
10.50%
发文量
650
审稿时长
1.9 months
期刊介绍:
The aim of Crystal Growth & Design is to stimulate crossfertilization of knowledge among scientists and engineers working in the fields of crystal growth, crystal engineering, and the industrial application of crystalline materials.
Crystal Growth & Design publishes theoretical and experimental studies of the physical, chemical, and biological phenomena and processes related to the design, growth, and application of crystalline materials. Synergistic approaches originating from different disciplines and technologies and integrating the fields of crystal growth, crystal engineering, intermolecular interactions, and industrial application are encouraged.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


