羟乙基淀粉共聚物纳米粒子通过抑制抗氧化系统促进光动力疗法和抗肿瘤免疫力
IF 11.9
1区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
光动力疗法(PDT)可产生大量活性氧(ROS),从而杀死肿瘤细胞并诱导抗肿瘤免疫。然而,细胞内的抗氧化系统,包括谷胱甘肽(GSH)系统和硫代氧化酶(Trx)系统,限制了ROS的积累,导致光动力疗法受到影响,免疫刺激不足。在此,我们设计了一种基于羟乙基淀粉(HES)的纳米药物PtHPs,其中共载光敏剂吡咯并卟啉a(PPa)和顺铂原药Pt-COOH(IV)(Pt (IV)),以抑制GSH和Trx抗氧化系统,实现强效PDT和抗肿瘤免疫反应。具体来说,将羟乙基淀粉与PPa和Pt(IV)偶联得到HES-PPa和HES-Pt,并通过乳化方法组装成纳米颗粒PtHPs,以达到PPa和Pt(IV)共同递送的目的。实验表明,PtHPs能降低GSH,抑制Trx系统,具有更好的细胞杀伤作用和ROS生成能力。皮下肿瘤模型显示,PtHPs 具有良好的安全性和肿瘤抑制效果。双侧肿瘤模型表明,PtHPs 可促进损伤相关分子模式的释放和树突状细胞的成熟,诱导 T 细胞介导的免疫反应,从而抑制原发性和远端肿瘤的生长。这项研究报告了一种新型铂基纳米药物,并提供了一种通过克服内在抗氧化系统来增强PDT疗法介导的抗肿瘤免疫的新策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
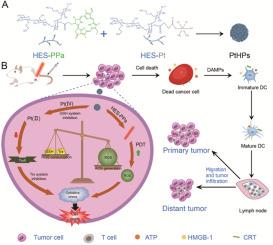
Hydroxyethyl starch conjugates co-assembled nanoparticles promote photodynamic therapy and antitumor immunity by inhibiting antioxidant systems
Photodynamic therapy (PDT) can produce high levels of reactive oxygen species (ROS) to kill tumor cells and induce antitumor immunity. However, intracellular antioxidant systems, including glutathione (GSH) system and thioredoxin (Trx) system, limit the accumulation of ROS, resulting in compromised PDT and insufficient immune stimulation. Herein, we designed a nanomedicine PtHPs co-loading photosensitizer pyropheophorbide a (PPa) and cisplatin prodrug Pt–COOH(IV) (Pt (IV)) based on hydroxyethyl starch (HES) to inhibit both GSH and Trx antioxidant systems and achieve potent PDT as well as antitumor immune responses. Specifically, HES-PPa and HES-Pt were obtained by coupling HES with PPa and Pt (IV), and assembled into nanoparticle PtHPs by emulsification method to achieve the purpose of co-delivery of PPa and Pt (IV). PtHPs improved PPa photostability while retaining PPa photodynamic properties. In vitro experiments showed that PtHPs reduced GSH, inhibited Trx system and had better cell-killing effect and ROS generation ability. Subcutaneous tumor models showed that PtHPs had good safety and tumor inhibition effect. Bilateral tumor models suggested that PtHPs promoted the release of damage-associated molecular patterns and the maturation of dendritic cells, induced T cell-mediated immune responses, and thus suppressed the growth of both primary and distal tumors. This study reports a novel platinum-based nanomedicine and provides a new strategy for boosting PDT therapy-mediated antitumor immunity by overcoming intrinsic antioxidant systems.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Asian Journal of Pharmaceutical Sciences
Pharmacology, Toxicology and Pharmaceutics-Pharmaceutical Science
CiteScore
18.30
自引率
2.90%
发文量
11
审稿时长
14 days
期刊介绍:
The Asian Journal of Pharmaceutical Sciences (AJPS) serves as the official journal of the Asian Federation for Pharmaceutical Sciences (AFPS). Recognized by the Science Citation Index Expanded (SCIE), AJPS offers a platform for the reporting of advancements, production methodologies, technologies, initiatives, and the practical application of scientific knowledge in the field of pharmaceutics. The journal covers a wide range of topics including but not limited to controlled drug release systems, drug targeting, physical pharmacy, pharmacodynamics, pharmacokinetics, pharmacogenomics, biopharmaceutics, drug and prodrug design, pharmaceutical analysis, drug stability, quality control, pharmaceutical engineering, and material sciences.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


