用于递送抗菌剂的可离子化脂质纳米载体
IF 11.1
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
摘要
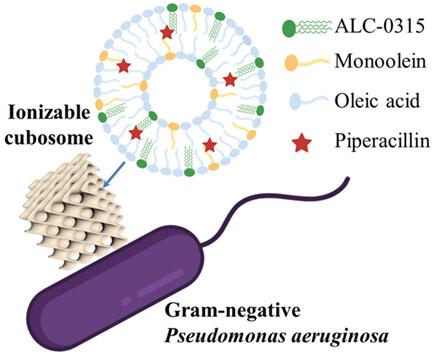
抗菌素耐药性(AMR)是一个全球性的健康危机,需要创新的解决方案。传统抗生素虽然在过去一个世纪中在抗击细菌感染方面发挥了关键作用,但面对不断演变的细菌防御机制,尤其是革兰氏阴性菌株,其疗效已大打折扣。本研究探讨了在纳米载体中加入两种可离子化脂质成分(一种阳离子,一种阴离子)的自组装可离子化脂质纳米颗粒(LNPs),用于广谱抗菌素哌拉西林(Piperacillin,Pip)的高级抗菌给药。在 LNPs 中加入阳离子可离子化脂质 ALC-0315(辉瑞-生物技术公司基于 mRNA 的 SARS-CoV-2 疫苗中公认的一种功能性脂质),可以利用同步辐射小角 X 射线散射、动态光散射和 2-(对甲苯胺基)-6-萘磺酸测定法研究介相转变、pH 值响应性和细菌感染部位酸性环境中的电离行为。加入另一种阴离子可离子化脂质油酸不仅能调节 LNPs 的理化性质,如尺寸、内相纳米结构和表面电荷,还能与 ALC-0315 协同增强抗菌效力,并有利于提高渗透性和与细菌膜的融合。这项研究介绍了在 LNPs 中定制可离子化脂质成分的策略,为抗菌治疗提供了一种新方法,有助于对抗 AMR。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Ionizable Lipid Containing Nanocarriers for Antimicrobial Agent Delivery
Antimicrobial resistance (AMR) poses a global health crisis demanding innovative solutions. Traditional antibiotics, though pivotal over the past century in combating bacterial infections, face diminished efficacy against evolving bacterial defense mechanisms, especially in Gram-negative strains. This study explores self-assembled ionizable lipid nanoparticles (LNPs) with the incorporation of two ionizable lipid components (one cationic, one anionic) in nanocarriers for advanced antimicrobial drug delivery of the broad-spectrum antibiotic Piperacillin (Pip). Incorporating cationic ionizable lipid ALC-0315, recognized as a functional lipid in the Pfizer-BioNTech mRNA-based SARS-CoV-2 vaccine, into LNPs allowed mesophase transition, pH responsiveness, and ionization behavior in acidic environments found in sites of bacterial infections, to be studied using synchrotron small angle X-ray scattering, dynamic light scattering, and a 2-(p-toluidino)-6-naphthalene sulfonic acid assay. Incorporating another anionic ionizable lipid, oleic acid not only modulates the LNPs’ physicochemical properties, such as size, internal phase nanostructure, and surface charge but also synergistically enhances the antimicrobial potency together with ALC-0315 with a benefit enhancing permeability and fusion with bacterial membranes. This study introduces a strategy for tailoring ionizable lipid compositions in LNPs, providing a new approach to antimicrobial treatment contributing to the fight against AMR.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
14.00
自引率
2.40%
发文量
0
期刊介绍:
Small Science is a premium multidisciplinary open access journal dedicated to publishing impactful research from all areas of nanoscience and nanotechnology. It features interdisciplinary original research and focused review articles on relevant topics. The journal covers design, characterization, mechanism, technology, and application of micro-/nanoscale structures and systems in various fields including physics, chemistry, materials science, engineering, environmental science, life science, biology, and medicine. It welcomes innovative interdisciplinary research and its readership includes professionals from academia and industry in fields such as chemistry, physics, materials science, biology, engineering, and environmental and analytical science. Small Science is indexed and abstracted in CAS, DOAJ, Clarivate Analytics, ProQuest Central, Publicly Available Content Database, Science Database, SCOPUS, and Web of Science.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


