氯化钾和磷酸氢二铵水溶液中抗坏血酸的粘度和电导特性分析
IF 2
3区 工程技术
Q3 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
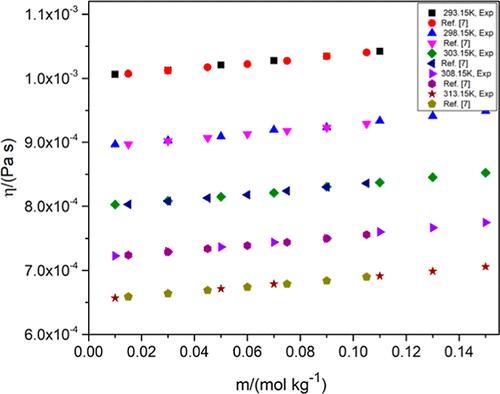
在本研究中,我们报告了维生素(即抗坏血酸(LAA))在水溶液和氯化钾(KCl)/磷酸氢二铵(DAP)二元水溶液中的实验粘度和电导特性,浓度范围为 0.01-0.15 mol kg-1,温度为 293.15、298.15、303.15、308.15 和 313.15 K。根据琼斯-多尔方程,将实验获得的动态粘度值和溶液摩尔浓度相关联,得出了福尔肯哈根系数 A、琼斯-多尔粘度 B 系数和 B 系数的温度导数,即(∂B/∂T)。此外,还计算了每摩尔溶剂的粘流活化自由能(Δμ10#)和每摩尔溶质的粘流活化自由能(Δμ20#)、每摩尔溶剂的粘流活化熵(ΔS20#)和粘流活化焓(ΔH20#)。此外,还测定了摩尔电导率 (Λm)、瓦尔登系数 (Λm0η0)、离子结合热力学参数和电荷移动活化能等电导参数。这些参数与 LAA 在水介质中与 KCl/DAP 作用时水的结构变化存在定性关联。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Analysis of Viscometric and Conductometric Properties of l-Ascorbic Acid in Aqueous Potassium Chloride and Diammonium Hydrogen Phosphate
In the present study, we report the experimental viscometric and conductometric properties of vitamins, namely, l-ascorbic acid (LAA), in aqueous and binary aqueous potassium chloride (KCl)/diammonium hydrogen phosphate (DAP) within the concentration range 0.01–0.15 mol kg–1 at T = 293.15, 298.15, 303.15, 308.15, and 313.15 K. The experimentally obtained dynamic viscosity values and the molarity of solutions have been correlated according to the Jones–Dole equation to derive the Falkenhagen coefficient A, Jones–Dole viscosity B-coefficient, and temperature derivative of B-coefficient, i.e., (∂B/∂T). Free energy of activation for viscous flow per mole of solvent (Δμ10#) and per mole of solute (Δμ20#), entropy, and enthalpy of activation for viscous flow per mole of solvent (ΔS20# and ΔH20#, respectively) have also been computed. Conductometric parameters like molar conductance (Λm), Walden factor (Λm0η0), thermodynamic parameters for ion association, and activation energy for charge mobility have been determined. These parameters were qualitatively correlated with changes in the structure of water that occurs when LAA interacts with KCl/DAP in aqueous media.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Chemical & Engineering Data
工程技术-工程:化工
CiteScore
5.20
自引率
19.20%
发文量
324
审稿时长
2.2 months
期刊介绍:
The Journal of Chemical & Engineering Data is a monthly journal devoted to the publication of data obtained from both experiment and computation, which are viewed as complementary. It is the only American Chemical Society journal primarily concerned with articles containing data on the phase behavior and the physical, thermodynamic, and transport properties of well-defined materials, including complex mixtures of known compositions. While environmental and biological samples are of interest, their compositions must be known and reproducible. As a result, adsorption on natural product materials does not generally fit within the scope of Journal of Chemical & Engineering Data.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


