实验性种子去甲基化后甲基组的长期变化及其与经常性水分胁迫的相互作用
IF 4.2
3区 生物学
Q1 PLANT SCIENCES
引用次数: 0
摘要
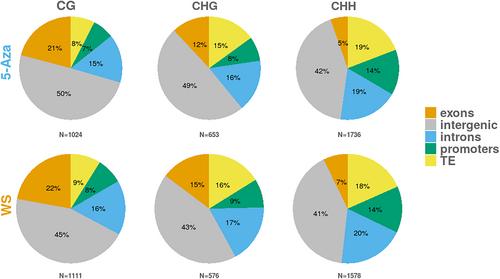
在全球亚热带和温带地区,干旱期的频率和长度都在增加。对水分胁迫的表观遗传学反应可能是植物抵御这些难以预测的挑战的关键。实验性 DNA 去甲基化以及胁迫因子的应用是揭示表观遗传学对植物胁迫反应的贡献的适当策略。我们在温室中分析了一年生地中海草本植物蝉衣草(Erodium cicutarium)成年植株在种子经 5-氮杂胞苷去甲基化和/或经常性水胁迫后叶片胞嘧啶甲基化的变化。我们使用亚硫酸氢盐 RADseq(BsRADseq)和新报道的蝉属草参考基因组,以 2 × 2 的因子设计描述了甲基化变化的特征,并控制了植物的亲缘关系。从长期来看,5-氮杂胞苷单独处理会导致单个胞嘧啶的低甲基化和高甲基化,其中 CG 上下文中的低甲基化程度很高。在对照条件下,干旱会导致除 CHH 上下文之外的所有甲基化水平降低。相比之下,经历过反复水胁迫并用 5-Azacytidine 处理过的植物基因组的 DNA 甲基化水平提高了约 5%。种子去甲基化与经常性干旱在全局和特异性胞嘧啶甲基化方面产生了非常显著的相互作用。大多数甲基化变化发生在基因区周围和可转座元件内。这些与基因相关的差异甲基化区域的注释包括一些在胁迫响应中可能发挥作用的基因(如 PAL、CDKC 和 ABCF),证实了在分子水平上对胁迫响应的表观遗传贡献。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Long-term methylome changes after experimental seed demethylation and their interaction with recurrent water stress in Erodium cicutarium (Geraniaceae)
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Plant Biology
生物-植物科学
CiteScore
8.20
自引率
2.60%
发文量
109
审稿时长
3 months
期刊介绍:
Plant Biology is an international journal of broad scope bringing together the different subdisciplines, such as physiology, molecular biology, cell biology, development, genetics, systematics, ecology, evolution, ecophysiology, plant-microbe interactions, and mycology.
Plant Biology publishes original problem-oriented full-length research papers, short research papers, and review articles. Discussion of hot topics and provocative opinion articles are published under the heading Acute Views. From a multidisciplinary perspective, Plant Biology will provide a platform for publication, information and debate, encompassing all areas which fall within the scope of plant science.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


