通过三组份苯并吡喃法异氰酸酯引导合成间位苯乙烯类化合物
IF 2.2
4区 化学
Q2 CHEMISTRY, ORGANIC
引用次数: 0
摘要
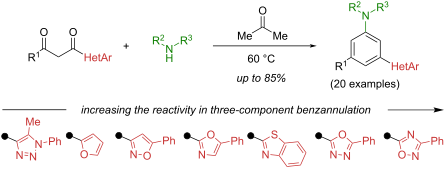
摘要 从杂环取代的 1,3-二酮中开发出了一种一锅三组份合成取代的元苯胺的方法。1,3- 二酮中杂环取代基的吸电子能力(可根据计算的哈米特常数估算)在所研究的反应中起着关键作用。所制备的一系列元香豆酰苯胺(分离收率为 21–85%)证明了所开发方法的合成实用性。Chem.2024, 20, 2208–2216. doi:10.3762/bjoc.20.188本文章由计算机程序翻译,如有差异,请以英文原文为准。
Heterocycle-guided synthesis of m-hetarylanilines via three-component benzannulation
Abstract
A one-pot three-component synthesis of substituted meta-hetarylanilines from heterocycle-substituted 1,3-diketones has been developed. The electron-withdrawing power of the heterocyclic substituent (which can be estimated on the basis of calculated Hammett constants) in the 1,3-diketone plays a pivotal role in the studied reaction. The series of meta-hetarylanilines prepared (21–85% isolated yield) demonstrates the synthetic utility of the developed method.
Beilstein J. Org. Chem. 2024, 20, 2208–2216. doi:10.3762/bjoc.20.188
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
4.90
自引率
3.70%
发文量
167
审稿时长
1.4 months
期刊介绍:
The Beilstein Journal of Organic Chemistry is an international, peer-reviewed, Open Access journal. It provides a unique platform for rapid publication without any charges (free for author and reader) – Platinum Open Access. The content is freely accessible 365 days a year to any user worldwide. Articles are available online immediately upon publication and are publicly archived in all major repositories. In addition, it provides a platform for publishing thematic issues (theme-based collections of articles) on topical issues in organic chemistry.
The journal publishes high quality research and reviews in all areas of organic chemistry, including organic synthesis, organic reactions, natural product chemistry, structural investigations, supramolecular chemistry and chemical biology.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


