gem-使用布氏酸/Bu4NBF4 或电生酸对碳-碳三键进行二氟化反应
IF 2.2
4区 化学
Q2 CHEMISTRY, ORGANIC
引用次数: 0
摘要
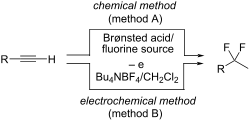
摘要利用 Brønsted 酸,如 Tf2NH 和 TfOH,结合 Bu4NBF4 作为氟源,进行了碳–碳三键的二氟化反应。含有炔基质的 Bu4NBF4/CH2Cl2 溶液的电化学氧化也能得到相应的二氟化宝石化合物(池内法)。电池外电解法也适用于炔烃的宝石二氟化。Chem.2024, 20, 2261–2269. doi:10.3762/bjoc.20.194本文章由计算机程序翻译,如有差异,请以英文原文为准。
gem-Difluorination of carbon–carbon triple bonds using Brønsted acid/Bu4NBF4 or electrogenerated acid
Abstract
gem-Difluorination of carbon–carbon triple bonds was conducted using Brønsted acids, such as Tf2NH and TfOH, combined with Bu4NBF4 as the fluorine source. The electrochemical oxidation of a Bu4NBF4/CH2Cl2 solution containing alkyne substrates could also give the corresponding gem-difluorinated compounds (in-cell method). The ex-cell electrolysis method was also applicable for gem-difluorination of alkynes.
Beilstein J. Org. Chem. 2024, 20, 2261–2269. doi:10.3762/bjoc.20.194
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
4.90
自引率
3.70%
发文量
167
审稿时长
1.4 months
期刊介绍:
The Beilstein Journal of Organic Chemistry is an international, peer-reviewed, Open Access journal. It provides a unique platform for rapid publication without any charges (free for author and reader) – Platinum Open Access. The content is freely accessible 365 days a year to any user worldwide. Articles are available online immediately upon publication and are publicly archived in all major repositories. In addition, it provides a platform for publishing thematic issues (theme-based collections of articles) on topical issues in organic chemistry.
The journal publishes high quality research and reviews in all areas of organic chemistry, including organic synthesis, organic reactions, natural product chemistry, structural investigations, supramolecular chemistry and chemical biology.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


