血液透析患者氯吡格雷药代动力学前瞻性试验
IF 5.7
2区 医学
Q1 UROLOGY & NEPHROLOGY
引用次数: 0
摘要
血液透析患者(HDPs)具有广泛的心血管风险。被广泛使用的抗血小板药物氯吡格雷通过细胞色素酶进行代谢活化,而尿毒症和慢性低度炎症可能会损害细胞色素酶的活性,而尿毒症和慢性低度炎症正是血液透析患者的典型症状。我们进行了一项前瞻性多中心研究,调查氯吡格雷在 HDPs 和健康志愿者(HVs)中的药代动力学和药效学。我们招募了长期接受氯吡格雷(75 毫克)和泮托拉唑(40 毫克)治疗的 HDP 患者。健康志愿者接受 300 毫克氯吡格雷的负荷剂量,然后每天一次,每次 75 毫克。静脉注射泮托拉唑(40 毫克),泮托拉唑是氯吡格雷的底物和探针药物。通过质谱法对血浆浓度进行量化。计算了药代动力学,并建立了群体药代动力学模型。主要终点是氯吡格雷活性代谢物的最大浓度。使用二磷酸腺苷诱导的全血聚集测定法测量血小板聚集。其中包括 17 种 HDP 和 16 种 HV。与 HV 相比,HDPs 中氯吡格雷活性代谢物的最大浓度明显较低(中位数 [四分位间范围] 12.2 [4.6-23.4] 与 24.7 [17.8-36.5] 纳克/毫升,0.02)。在 HDPs 中,氯吡格雷活性代谢物与原药的最大浓度比降低了 8.5 倍,通过群体药代动力学模型发现,氯吡格雷清除率(包括氯吡格雷活性代谢物的形成)降低了 82.7%。根据先前的研究,120 分钟时二磷酸腺苷诱导的血小板聚集在 HDPs 中明显高于 HVs(中位数[四分位间范围]:26 U [14 U-43 U] vs. 12 U [11 U-18 U],0.004)。泮托拉唑的终末半衰期在 HDPs 中比在 HVs 中高 1.7 倍。我们的数据表明,在活性较低的情况下,HDPs 中氯吡格雷的代谢发生了改变,这可能对该酶代谢的其他物质产生影响。本文章由计算机程序翻译,如有差异,请以英文原文为准。
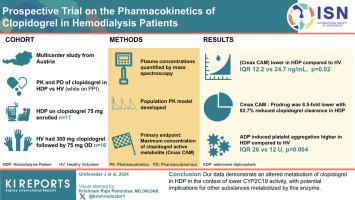
Prospective Trial on the Pharmacokinetics of Clopidogrel in Hemodialysis Patients
Introduction
Hemodialysis patients (HDPs) exhibit extensive cardiovascular risk. The widely prescribed anti-platelet agent clopidogrel is metabolically activated by cytochrome enzymes, which may be impaired by uremia and chronic low-grade inflammation, typically present in HDPs. We conducted a prospective multicenter study to investigate the pharmacokinetics and pharmacodynamics of clopidogrel in HDPs and healthy volunteers (HVs).
Methods
We enrolled HDPs receiving long-term clopidogrel (75 mg) and pantoprazole treatment (40 mg). Healthy volunteers received a loading dose of 300 mg clopidogrel, followed by 75 mg once daily. Pantoprazole, a substrate and probe drug of CYP2C19, was administered intravenously (40 mg). Plasma concentrations were quantified by mass spectrometry. Pharmacokinetics were calculated, and a population pharmacokinetic model was developed. The primary endpoint was the maximum concentration of clopidogrel’s active metabolite. Platelet aggregation was measured using adenosine diphosphate-induced whole-blood aggregometry.
Results
Seventeen HDPs and 16 HVs were included. The maximum concentration of clopidogrel’s active metabolite was significantly lower in HDPs compared to HVs (median [interquartile range] 12.2 [4.6–23.4] vs. 24.7 [17.8–36.5] ng/ml, P = 0.02). The maximum concentration ratio of clopidogrel’s active metabolite to prodrug was 8.5-fold lower in HDPs, and an 82.7% reduced clopidogrel clearance, including clopidogrel’s active metabolite formation, was found using population pharmacokinetic modeling. From previous studies, adenosine diphosphate-induced platelet aggregation at 120 minutes was significantly higher in HDPs than in HVs (median [interquartile range]: 26 U [14 U–43 U] vs. 12 U [11 U–18 U], P = 0.004. Pantoprazole terminal half-life was ∼1.7-fold higher in HDPs compared to HVs.
Conclusion
Our data demonstrate an altered metabolism of clopidogrel in HDPs in the context of lower CYP2C19 activity, with potential implications for other substances metabolized by this enzyme.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Kidney International Reports
Medicine-Nephrology
CiteScore
7.70
自引率
3.30%
发文量
1578
审稿时长
8 weeks
期刊介绍:
Kidney International Reports, an official journal of the International Society of Nephrology, is a peer-reviewed, open access journal devoted to the publication of leading research and developments related to kidney disease. With the primary aim of contributing to improved care of patients with kidney disease, the journal will publish original clinical and select translational articles and educational content related to the pathogenesis, evaluation and management of acute and chronic kidney disease, end stage renal disease (including transplantation), acid-base, fluid and electrolyte disturbances and hypertension. Of particular interest are submissions related to clinical trials, epidemiology, systematic reviews (including meta-analyses) and outcomes research. The journal will also provide a platform for wider dissemination of national and regional guidelines as well as consensus meeting reports.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


