在糖尿病肾病中,TRPC6-Calpain-1 轴通过抑制丝裂吞噬促进肾小管间质炎症
IF 5.7
2区 医学
Q1 UROLOGY & NEPHROLOGY
引用次数: 0
摘要
肾小管间质炎症是预测糖尿病肾病(DKD)进展的有效指标。有丝分裂异常是导致肾小管损伤的最重要因素之一。然而,人们对糖尿病肾病患者有丝分裂异常介导的肾小管间质炎症的确切分子机制仍然知之甚少。本研究建立了链脲佐菌素诱导的 DKD 小鼠模型,并以高糖(HG)处理的 HK-2 细胞作为模型。通过给药尿石素 A(UA)来调节肾小管有丝分裂。通过基因干预和.NET技术探讨了瞬态受体电位阳离子通道C亚家族成员6(TRPC6)的功能效应。我们发现,DKD 肾小管间质炎症与抑制有丝分裂密切相关,而抑制有丝分裂是由 PINK1/parkin 通路紊乱介导的。激活有丝分裂可明显减轻肾小管损伤和肾小管间质炎症。研究还发现,TRPC6在DKD中明显增高,并通过激活钙蛋白酶-1在抑制有丝分裂过程中发挥重要作用。敲除 TRPC6 可部分逆转有丝分裂异常,从而减轻肾小管损伤和肾小管间质炎症。最后,我们发现 BAPTA(一种特异性钙螯合剂)或 calpeptin(一种特异性钙蛋白酶-1 抑制剂)可阻断肾小管 TRPC6 介导的有丝分裂抑制。我们的研究揭示,TRPC6-钙蛋白酶-1 轴通过抑制有丝分裂促进了 DKD 肾小管间质炎症。本文章由计算机程序翻译,如有差异,请以英文原文为准。
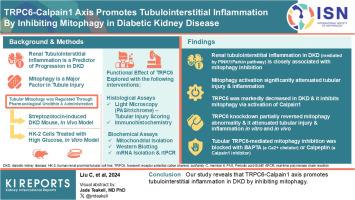
TRPC6-Calpain-1 Axis Promotes Tubulointerstitial Inflammation by Inhibiting Mitophagy in Diabetic Kidney Disease
Introduction
Renal tubulointerstitial inflammation represents an effective indicator for predicting the progression of diabetic kidney disease (DKD). Mitophagy abnormality is 1 of the most important factors involved in tubule injury. However, the exact molecular mechanism underlying mitophagy abnormality-mediated tubulointerstitial inflammation in DKD remains poorly understood.
Methods
In this study, a streptozotocin-induced DKD mouse model was established and HK-2 cells treated with high glucose (HG) served as an in vitro model. Tubular mitophagy was regulated through pharmacological urolithin A (UA) administration. The functional effect of the transient receptor potential cation channel, subfamily C, member 6 (TRPC6) was explored using genetic interventions in vivo and in vitro.
Results
We found that renal tubulointerstitial inflammation in DKD was closely associated with mitophagy inhibition, which was mediated by disturbance of PINK1/Parkin pathway. Mitophagy activation significantly attenuated tubular injury and tubulointerstitial inflammation. Further, it was found that TRPC6 was markedly increased in DKD and played an essential role in mitophagy inhibition by activating calpain-1. Knockdown of Trpc6 partially reversed mitophagy abnormality and consequently attenuated tubular injury and tubulointerstitial inflammation in vivo and in vitro. Finally, we found that tubular TRPC6-mediated mitophagy inhibition was blocked with BAPTA (a specific Ca2+ chelator) or calpeptin (a specific calpain-1 inhibitor).
Conclusion
Our study reveals that TRPC6-calpain-1 axis promotes tubulointerstitial inflammation in DKD by inhibiting mitophagy.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Kidney International Reports
Medicine-Nephrology
CiteScore
7.70
自引率
3.30%
发文量
1578
审稿时长
8 weeks
期刊介绍:
Kidney International Reports, an official journal of the International Society of Nephrology, is a peer-reviewed, open access journal devoted to the publication of leading research and developments related to kidney disease. With the primary aim of contributing to improved care of patients with kidney disease, the journal will publish original clinical and select translational articles and educational content related to the pathogenesis, evaluation and management of acute and chronic kidney disease, end stage renal disease (including transplantation), acid-base, fluid and electrolyte disturbances and hypertension. Of particular interest are submissions related to clinical trials, epidemiology, systematic reviews (including meta-analyses) and outcomes research. The journal will also provide a platform for wider dissemination of national and regional guidelines as well as consensus meeting reports.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


