肾移植前基于达拉单抗的脱敏治疗的开放标签 1/2期研究
IF 8.3
2区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
摘要
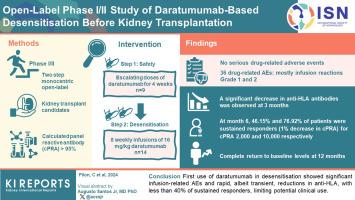
抗CD38单克隆抗体daratumumab能在肾移植前诱导致敏患者溶解产生抗体的浆细胞,其安全性和益处仍有待确定。我们开展了一项分两个阶段(1和2)的单中心开放标签研究,以评估达拉土穆单抗对肾移植候选者第6个月(M6)的安全性和疗效,这些候选者的计算小组反应性抗体(cPRA)> 95%。在第一阶段(安全性),我们使用了4周递增剂量的daratumumab。cPRA 10,000的计算只考虑平均荧光强度(MFI)大于10,000的抗人类白细胞抗原(HLA)抗体。第一阶段共招募了 9 名患者,第二阶段招募了 14 名患者。安全性分析显示,有4例严重的非治疗突发不良事件(non-TEAEs),36例轻微的TEAEs,主要是输液相关的1级和2级反应(导致2例暂时停药),但没有严重的TEAEs。第3个月(M3)观察到抗-HLA抗体显著降低,cPRA 10,000(=0.003)、抗-HLA数量(<0.001)、最大MFI(MFI max)(=0.053)和MFI总和(MFI sum)(<0.001),第12个月(M12)完全恢复到基线水平。在第 6 个月,分别有 46.15% (19.22%-74.87%)和 76.92% (46.19%-94.96%)的患者对 cPRA 2000 和 10,000 出现持续应答(cPRA 下降 1%)。在第 1 个月(M1),免疫细胞(T-reg、CD8 + TEMRA、CD19 + CD138 + B 细胞和 NK 细胞)显著减少。在M3时,其他抗体明显下降,但在M12时,除γ球蛋白外,其他抗体恢复到基线水平,且未出现任何感染性并发症。达拉土单抗首次用于脱敏治疗时出现了输注相关不良(AEs)事件,抗HLA抗体迅速下降,但只是短暂的,持久应答者不足40%,这限制了其潜在的临床应用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Open-Label Phase 1/2 Study of Daratumumab-Based Desensitization Before Kidney Transplantation
Introduction
The safety and benefit of the anti-CD38 monoclonal antibody daratumumab, which induces lysis of antibody-producing plasma cells in sensitized patients prior to kidney transplantation, remain to be determined.
Methods
A 2-phase (1 and 2), monocentric open-label study was conducted to evaluate the month 6 (M6) safety and efficacy of daratumumab in kidney transplant candidates with calculated panel reactive antibody (cPRA) > 95%. In the first (safety) phase, we used 4-weekly escalating doses of daratumumab. Phase 2 tested desensitization with 8 weekly infusions of 16 mg/kg daratumumab. cPRA 10,000 was calculated considering only human leukocyte antigen (HLA) antibodies with mean fluorescence intensity (MFI) of > 10,000.
Results
Nine patients were enrolled in phase 1 and 14 in phase 2. Safety analysis showed 4 serious non-treatment-emergent adverse events (non-TEAEs), 36 mild TEAEs, mostly infusion-related reactions, grade 1 and 2 (causing 2 temporary drug discontinuations), but no serious TEAEs. Significant reductions in anti-HLA antibodies were observed at month 3 (M3), with cPRA 10,000 (P = 0.003), number of anti-HLA (P < 0.001), maximum MFI (MFI max) (P = 0.053), and the sum of MFI (MFI sum) (P < 0.001), with complete return to baseline levels at month 12 (M12). At M6, 46.15% (19.22%–74.87%) and 76.92% (46.19%–94.96%) of patients showed sustained response (1% decrease in cPRA) for cPRA 2000 and 10,000, respectively. At month 1 (M1), immune cells (T-reg, CD8 + TEMRA, CD19 + CD138 + B cells, and NK cells) significantly decreased. At M3, other antibodies decreased significantly, but returned to baseline levels at M12, except for gamma globulins, without any infectious complications.
Conclusion
The first use of daratumumab in desensitization demonstrated infusion-related adverse (AEs) events and rapid, albeit transient, reductions in anti-HLA antibodies, with less than 40% of durable responders, limiting its potential clinical use.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Applied Materials & Interfaces
工程技术-材料科学:综合
CiteScore
16.00
自引率
6.30%
发文量
4978
审稿时长
1.8 months
期刊介绍:
ACS Applied Materials & Interfaces is a leading interdisciplinary journal that brings together chemists, engineers, physicists, and biologists to explore the development and utilization of newly-discovered materials and interfacial processes for specific applications. Our journal has experienced remarkable growth since its establishment in 2009, both in terms of the number of articles published and the impact of the research showcased. We are proud to foster a truly global community, with the majority of published articles originating from outside the United States, reflecting the rapid growth of applied research worldwide.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


