丙氨酰还原、还原偶联和有机腈的 C-H 异构化
IF 2.9
3区 化学
Q2 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
摘要
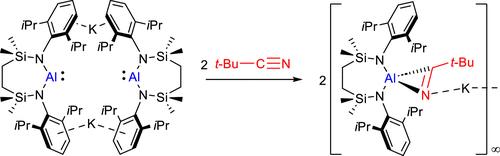
我们研究了[{SiNDipp}AlK]2({SiNDipp} = {CH2SiMe2N(Dipp)}2;Dipp = 2,6-i-Pr2C6H3)这种铝酰钾与有机腈的反应行为。与早先对电荷中性 Al(I) 物种与多键小分子反应性的研究一样,所有反应的第一步都涉及[2 + 1]环加成和生成[η2-C═N-Al]氧化铝偶氮环丙烷单元。在邻甲苯基和间甲苯基取代的芳基腈的情况下,这种物质在动力学上过于易变,无法将其分离,只能通过 Al-C/C≡N 直接插入进行 C-C 偶联,生成氧化铝重氮丁二烯衍生物。与此相反,烷基腈的立体性增加,对所形成产物的性质产生了明显的影响。与提出的顺序途径相一致,[{SiNDipp}AlK]2 与 t-BuCN 的反应提供了一种可分离的氧化铝环丙烷,这种环丙烷在动力学上不易与进一步的腈当量发生反应。虽然 i-PrCN 的使用降低了烷基腈的立体需求,再次促进了 C-C 键的形成,但由此产生的氧化铝-二氮杂双烯二酮分子对铝中心的挤压似乎超出了动力学可行性的极限,导致一个 C-异丙基取代基发生了不寻常的 2 倍 C-H 到 N-H 异构化,并分离出 1-氧化铝-2,5-二氮杂双烯二酮结构。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Alumanyl Reduction, Reductive Coupling and C–H Isomerization of Organic Nitriles
The behavior of the potassium alumanyl, [{SiNDipp}AlK]2 ({SiNDipp} = {CH2SiMe2N(Dipp)}2; Dipp = 2,6-i-Pr2C6H3), toward organic nitriles has been investigated. In common with earlier studies of the reactivity of charge neutral Al(I) species with multiply bonded small molecules, it is suggested that the initial step in all the reactions involves [2 + 1] cycloaddition and the generation of an [η2–C═N–Al] alumina azacyclopropane unit. In the cases of o- and m-tolyl-substituted aryl nitriles, this species is too kinetically labile to allow its isolation and undergoes C–C coupling via immediate Al–C/C≡N insertion to yield the alumina diazabutadiene derivatives. In contrast, the increased steric profile of alkyl nitriles imposes a marked influence on the nature of the products formed. Consistent with the proposed sequential pathway, reaction of [{SiNDipp}AlK]2 with t-BuCN provides an isolable alumina cyclopropane species that is kinetically resistant to onward reaction with a further nitrile equivalent. While reduction in the alkyl nitrile steric demands by use of i-PrCN again facilitates C–C bond formation, the crowding of the Al center by the resultant alumina-diazabutadienediide moiety appears to be beyond the limit of kinetic viability, resulting in an unusual 2-fold C–H to N–H isomerization from one of the C-iso-propyl substituents and the isolation of a 1-alumina-2,5-diazabutadiene structure.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organometallics
化学-无机化学与核化学
CiteScore
5.60
自引率
7.10%
发文量
382
审稿时长
1.7 months
期刊介绍:
Organometallics is the flagship journal of organometallic chemistry and records progress in one of the most active fields of science, bridging organic and inorganic chemistry. The journal publishes Articles, Communications, Reviews, and Tutorials (instructional overviews) that depict research on the synthesis, structure, bonding, chemical reactivity, and reaction mechanisms for a variety of applications, including catalyst design and catalytic processes; main-group, transition-metal, and lanthanide and actinide metal chemistry; synthetic aspects of polymer science and materials science; and bioorganometallic chemistry.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


