调整偶氮膦配体的电子特性及其在无碱转移加氢催化中的应用
IF 2.5
3区 化学
Q2 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
摘要
设计和调整新配体对于开启过渡金属中心的新反应活性至关重要。最近,偶氮膦成为钌琴凳配合物中一类新的 1,3-P,N配体。这项研究表明,偶氮膦的合成可以容忍带有强电子供体和强电子吸附对位 R 基团的 N 芳基取代基,而且 R 基团的性质会影响偶氮膦的光谱和结构特性,这些特性可以通过核磁共振光谱、紫外可见光谱、单晶 X 射线衍射和 DFT 研究来测量。通过分析相应偶氮膦硒化物的 1JP-Se 耦合常数,可以看出偶氮膦是相对较弱的膦供体,但供体特性可以在这一化学空间范围内进行微调。研究人员制备了单齿和双齿 Ru-叠氮膦配合物,并首次将其用作催化剂。研究发现,Ru-偶氮膦配合物能促进苯乙酮向 1-苯乙醇的转移氢化,而不需要苛刻的碱添加剂,并且双齿配合物比单齿类似物更活跃。本文章由计算机程序翻译,如有差异,请以英文原文为准。
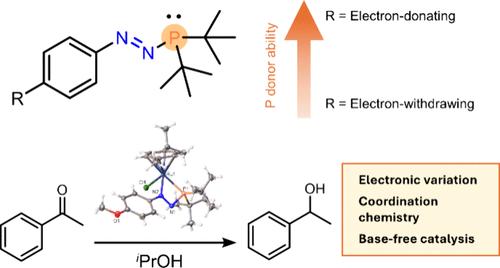
Tuning the Electronic Properties of Azophosphines as Ligands and Their Application in Base-Free Transfer Hydrogenation Catalysis
The design and tuning of new ligands is crucial for unlocking new reactivity at transition metal centers. Azophosphines have recently emerged as a new class of 1,3-P,N ligands in ruthenium piano-stool complexes. This work shows that the azophosphine synthesis can tolerate N-aryl substituents with strongly electron-donating and electron-withdrawing para-R groups and that the nature of this R group can affect the spectroscopic and structural properties of the azophosphines, as measured by NMR spectroscopy, UV–vis spectroscopy, single-crystal X-ray diffraction, and DFT studies. Azophosphines are shown to be relatively weak phosphine donors, as shown by analysis of the 1JP–Se coupling constants of the corresponding azophosphine selenides, but the donor properties can be fine tuned within this area of chemical space. Monodentate and bidentate Ru–azophosphine complexes were prepared, and their first use as a catalyst was probed. The Ru–azophosphine complexes were found to promote the transfer hydrogenation of acetophenone to 1-phenylethanol without the requirement of a harsh base additive, and the bidentate complex was more active than the monodentate analogue.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organometallics
化学-无机化学与核化学
CiteScore
5.60
自引率
7.10%
发文量
382
审稿时长
1.7 months
期刊介绍:
Organometallics is the flagship journal of organometallic chemistry and records progress in one of the most active fields of science, bridging organic and inorganic chemistry. The journal publishes Articles, Communications, Reviews, and Tutorials (instructional overviews) that depict research on the synthesis, structure, bonding, chemical reactivity, and reaction mechanisms for a variety of applications, including catalyst design and catalytic processes; main-group, transition-metal, and lanthanide and actinide metal chemistry; synthetic aspects of polymer science and materials science; and bioorganometallic chemistry.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


