从矿尘中释放的 HOSO2:沙尘暴期间二氧化硫异质氧化的新渠道
IF 2.9
3区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
矿物尘埃已被认为是一种新兴的自由基来源,可能会对环境污染物的归宿产生重大影响。虽然最近已经明确了水性粉尘表面产生羟基(OH)自由基的途径,但对其他自由基产生的途径却知之甚少。在此,我们提出了二氧化硫(SO2)异质氧化过程中,α-Fe2O3(一种无处不在的铁矿物)水表面产生羟基磺酰基(HOSO2)自由基的新渠道。反应力场分子动力学(ReaxFF-MD)模拟和密度泛函理论(DFT)计算表明,在表面水层存在的情况下,α-Fe2O3 表面结合的 OH 基团可将吸附的 SO2 氧化成 HOSO2 自由基。HOSO2 自由基可从粉尘表面释放出来,随后促成气态硫酸 (H2SO4) 的形成。我们的动力学模型显示,尽管大多数二氧化硫会通过水性尘埃表面的竞争反应转化为界面硫酸盐,但沙尘暴期间表面结合的大量羟基和尘埃颗粒的高浓度可能会产生大量气态 H2SO4。从 HOSO2 释放通道产生的气态 H2SO4 可能与传统气相 OH 氧化途径产生的 H2SO4 水平相当。这项研究表明,矿物产生的自由基可能在转化大气污染物方面发挥关键作用,从而调节矿尘对当地和区域空气质量的影响。本文章由计算机程序翻译,如有差异,请以英文原文为准。
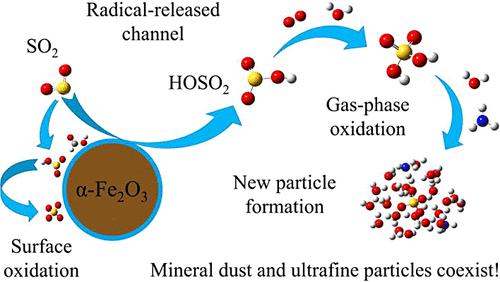
HOSO2 Released from Mineral Dust: A Novel Channel of Heterogeneous Oxidation of Sulfur Dioxide during Dust Storms
Mineral dust has been recognized as an emerging source of radicals that may significantly influence the fate of environmental pollutants. While the production of hydroxyl (OH) radicals from an aqueous dust surface has been recently clarified, other radical-produced channels have been little understood. Here, we propose a novel channel of hydroxysulfonyl (HOSO2) radical production from the aqueous surface of α-Fe2O3, a ubiquitous iron mineral, during the heterogeneous oxidation of sulfur dioxide (SO2). The reactive force field molecular dynamic (ReaxFF-MD) simulations and the density functional theory (DFT) calculations disclosed that the OH groups bound to the α-Fe2O3 surface can oxidize the adsorbed SO2 into HOSO2 radicals in the presence of the surface water layer. The HOSO2 radical can be released from the dust surface and subsequently contribute to the gaseous sulfuric acid (H2SO4) formation. Our kinetic modeling revealed that, despite most SO2 converting to interfacial sulfate through competing reactions on the aqueous dust surface, the abundant surface-bound OH groups and high dust particle concentration during dust storms may enable substantial gaseous H2SO4 production. The level of gaseous H2SO4 production from the HOSO2-released channel is likely comparable to that from the traditional gas-phase OH oxidation pathway. This study demonstrates that mineral-produced radicals may play a critical role in transforming atmospheric pollutants and hence modulate the impacts of mineral dust on local and regional air quality.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Earth and Space Chemistry
Earth and Planetary Sciences-Geochemistry and Petrology
CiteScore
5.30
自引率
11.80%
发文量
249
期刊介绍:
The scope of ACS Earth and Space Chemistry includes the application of analytical, experimental and theoretical chemistry to investigate research questions relevant to the Earth and Space. The journal encompasses the highly interdisciplinary nature of research in this area, while emphasizing chemistry and chemical research tools as the unifying theme. The journal publishes broadly in the domains of high- and low-temperature geochemistry, atmospheric chemistry, marine chemistry, planetary chemistry, astrochemistry, and analytical geochemistry. ACS Earth and Space Chemistry publishes Articles, Letters, Reviews, and Features to provide flexible formats to readily communicate all aspects of research in these fields.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


