开发粘蛋白和癌细胞样本中微量酸性 O-糖分析方法
IF 3.6
2区 生物学
Q1 BIOCHEMICAL RESEARCH METHODS
引用次数: 0
摘要
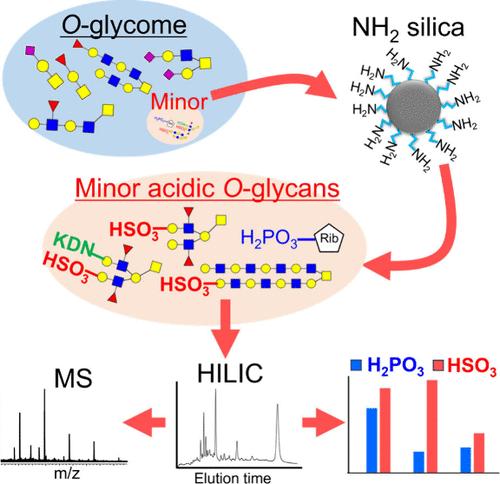
硫酸化聚糖和磷酸化聚糖等微酸性聚糖只占生物糖元的一小部分,因此对它们的分析是一项相当大的挑战。在这项研究中,我们开发了一种分析生物样本中次要酸性 O 型聚糖的技术。首先,确定了从蛋白质中释放 O 型糖的有效反应条件。接着,我们建立了一种使用 NH2 旋转柱回收微酸性聚糖的高通量方法。我们使用粘蛋白样品对所建立方法的性能进行了评估,并在牛颌下腺粘蛋白和猪胃粘蛋白中成功检测到了硫酸化的 O 型聚糖。我们还分析了培养癌细胞中的次酸性 O 型糖。除了三ucosylated硫酸化O型聚糖和二硫化O型聚糖外,我们还在LS174T细胞中检测到了含有KDN的硫酸化O型聚糖。LS174T 细胞中硫酸化聚糖的相对含量几乎是其他细胞的 10 倍。此外,在 MKN45 细胞中还检测到分子量为 3500 的大分子聚乳糖胺硫酸化 O 型聚糖。有趣的是,在本研究使用的所有细胞中都观察到了可能与丝氨酸/苏氨酸结合的磷酸化核糖。因此,我们所建立的分析方法可以分析现有糖化学分析方法无法检测到的微小酸性 O-聚糖。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Developing Method for Minor Acidic O-Glycan Analysis in Mucin and Cancer Cell Samples
Minor acidic glycans, such as sulfated and phosphorylated glycans, constitute only a small fraction of biological glycome, making their analysis a considerable challenge. In this study, we developed a technique to analyze minor acidic O-glycans in biological samples. First, efficient reaction conditions for the release of O-glycans from the proteins were determined. Next, a high-throughput method was established for the recovery of minor acidic glycans using NH2 spin columns. The performance of the established method was evaluated using mucin samples, and sulfated O-glycans were successfully detected in bovine submaxillary gland mucin and porcine stomach mucin. We also analyzed the minor acidic O-glycans in cultured cancer cells. In addition to trifucosylated sulfated O-glycans and disulfated O-glycans, sulfated O-glycans with KDN were detected in LS174T cells. The relative amount of sulfated glycans in LS174T cells was almost 10-fold higher than that in the other cells. Moreover, a large polylactosamine-type sulfated O-glycan with a molecular weight >3500 was detected in MKN45 cells. Interestingly, phosphorylated ribose, possibly bound to serine/threonine, was observed in all the cells used in this study. Thus, our established analytical method allows for the analysis of minor acidic O-glycans that cannot be detected using existing glycomics methods.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Proteome Research
生物-生化研究方法
CiteScore
9.00
自引率
4.50%
发文量
251
审稿时长
3 months
期刊介绍:
Journal of Proteome Research publishes content encompassing all aspects of global protein analysis and function, including the dynamic aspects of genomics, spatio-temporal proteomics, metabonomics and metabolomics, clinical and agricultural proteomics, as well as advances in methodology including bioinformatics. The theme and emphasis is on a multidisciplinary approach to the life sciences through the synergy between the different types of "omics".
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


