单萜吲哚生物碱低聚物组装的结构多样性和化学逻辑
IF 10.6
1区 化学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
覆盖范围:至 2024 年本文章由计算机程序翻译,如有差异,请以英文原文为准。
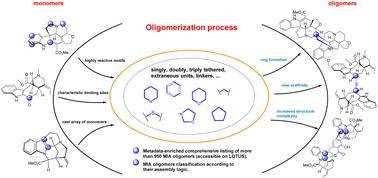
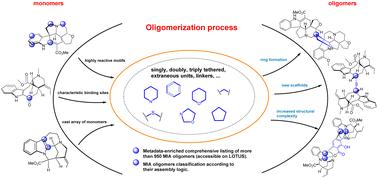
Structural diversity and chemical logic underlying the assembly of monoterpene indole alkaloids oligomers†‡
Covering: up to 2024
This review aims to draw a parallel between all known oligomers of monoterpene indole alkaloids (MIAs) by illustrating the chemical logic underlying their assembly. For this purpose, oligomeric MIAs were first comprehensively listed and organized according to the names of the backbones of their constitutive monomers and the binding sites. From this extensive list, an oligomer network was generated and unprecedented MIA statistics were mined and shared herein. Subsequently, oligomeric MIAs were categorized according to the number of connections instigated between their monomeric components (single, double, triple, and mixed tethering), then subdivided according to the uniqueness or combination of oligomerization assembly reactions. This effort outlined oligomerization trends in a scaffold-specific manner, and established binding reactivity patterns facilitating the comprehension of the associated biosynthetic processes. At last, this review illustrates a unique initiative in crafting a comprehensive repository of machine-readable metadata for MIA oligomers that could be leveraged for chemoinformatic purposes.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Natural Product Reports
化学-生化与分子生物学
CiteScore
21.20
自引率
3.40%
发文量
127
审稿时长
1.7 months
期刊介绍:
Natural Product Reports (NPR) serves as a pivotal critical review journal propelling advancements in all facets of natural products research, encompassing isolation, structural and stereochemical determination, biosynthesis, biological activity, and synthesis.
With a broad scope, NPR extends its influence into the wider bioinorganic, bioorganic, and chemical biology communities. Covering areas such as enzymology, nucleic acids, genetics, chemical ecology, carbohydrates, primary and secondary metabolism, and analytical techniques, the journal provides insightful articles focusing on key developments shaping the field, rather than offering exhaustive overviews of all results.
NPR encourages authors to infuse their perspectives on developments, trends, and future directions, fostering a dynamic exchange of ideas within the natural products research community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


