组装源自干细胞的人类着床后胚胎模型
IF 13.1
1区 生物学
Q1 BIOCHEMICAL RESEARCH METHODS
引用次数: 0
摘要
人类胚胎发育在植入阶段所依赖的胚胎和胚外组织相互作用目前尚不清楚。我们已经生成了一个多能干细胞衍生模型,该模型可模仿着床前发育的各个方面,从而可进行人类胚胎不可能进行的实验。在人类胚胎干细胞(ES)中激活胚外系特异性转录因子GATA6和SOX17(胚下细胞因子)或GATA3和TFAP2C(编码AP2γ;滋养层细胞因子),可推动向胚外样细胞转化。当与野生型 ES 细胞结合时,在没有外源因子的情况下会形成自组织胚胎样结构,称为人类可诱导胚胎(hiEmbryoids)。hiEmbryoids的上胚层样域会根据胚外分泌的细胞外基质和形态发生因子进行极化和分化。胚外间充质、羊膜和原始生殖细胞在 hiEmbryoids 中逐步分化。在建立稳定的可诱导 ES 系并将 ES 细胞转化为 RSeT 培养基后,该方案需要 7-10 天才能生成高胚胎性细胞。具有干细胞培养基础知识的研究人员都可以进行干胚胎的生成。本文章由计算机程序翻译,如有差异,请以英文原文为准。
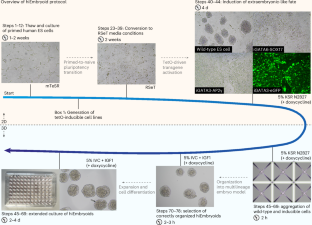

Assembly of a stem cell-derived human postimplantation embryo model
The embryonic and extraembryonic tissue interactions underlying human embryogenesis at implantation stages are not currently understood. We have generated a pluripotent stem cell-derived model that mimics aspects of peri-implantation development, allowing tractable experimentation otherwise impossible in the human embryo. Activation of the extraembryonic lineage-specific transcription factors GATA6 and SOX17 (hypoblast factors) or GATA3 and TFAP2C (encoding AP2γ; trophoblast factors) in human embryonic stem (ES) cells drive conversion to extraembryonic-like cells. When combined with wild-type ES cells, self-organized embryo-like structures form in the absence of exogenous factors, termed human inducible embryoids (hiEmbryoids). The epiblast-like domain of hiEmbryoids polarizes and differentiates in response to extraembryonic-secreted extracellular matrix and morphogen cues. Extraembryonic mesenchyme, amnion and primordial germ cells are specified in hiEmbryoids in a stepwise fashion. After establishing stable inducible ES lines and converting ES cells to RSeT culture media, the protocol takes 7–10 d to generate hiEmbryoids. Generation of hiEmbryoids can be performed by researchers with basic expertise in stem cell culture. Protocol for the generation of a stem cell-derived human postimplantation embryo model by the combination of embryonic and transgene-induced extraembryonic-like cells.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Protocols
生物-生化研究方法
CiteScore
29.10
自引率
0.70%
发文量
128
审稿时长
4 months
期刊介绍:
Nature Protocols focuses on publishing protocols used to address significant biological and biomedical science research questions, including methods grounded in physics and chemistry with practical applications to biological problems. The journal caters to a primary audience of research scientists and, as such, exclusively publishes protocols with research applications. Protocols primarily aimed at influencing patient management and treatment decisions are not featured.
The specific techniques covered encompass a wide range, including but not limited to: Biochemistry, Cell biology, Cell culture, Chemical modification, Computational biology, Developmental biology, Epigenomics, Genetic analysis, Genetic modification, Genomics, Imaging, Immunology, Isolation, purification, and separation, Lipidomics, Metabolomics, Microbiology, Model organisms, Nanotechnology, Neuroscience, Nucleic-acid-based molecular biology, Pharmacology, Plant biology, Protein analysis, Proteomics, Spectroscopy, Structural biology, Synthetic chemistry, Tissue culture, Toxicology, and Virology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


