合成新型喹唑啉酮-三杂环杂化物作为脲酶和胃酸的双重抑制剂
IF 2.2
4区 化学
Q3 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
合成了含有喹唑啉酮、噻吩和噻二唑/噻唑烷酮结构的新的三杂环化合物,并通过傅立叶变换红外光谱、1H-NMR 和 13C-NMR 光谱数据对其进行了表征。评估了新化合物对脲酶和乙酰胆碱酯酶的抑制活性。特别是化合物 5a;3-[(5-(苯基氨基)-1,3,4-噻二唑-2-基]甲基-2-(噻吩-3-基甲基)喹唑啉-4(3H)-酮对尿素酶的抑制效果最好,为 13.它对乙酰胆碱酯酶的抑制效果也最好,IC50 值为 20.30 ± 0.15 µg/mL。本文章由计算机程序翻译,如有差异,请以英文原文为准。
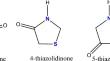
Synthesis of novel quinazolinone-triheterocyclic hybrides as dual inhibition of urease and ache
New triheteroyclic compounds containing quinazolinone, thiophene, andthiadiazole /thiazolidinone structureswere synthesized and characterized by FT-IR, 1H–NMR, and13C–NMRspectral data. The new compounds’ inhibitory activities on urease and acetylcholinesterase were assessed. All triheterocyclic compounds with thiadiazole ring have urease and acetylcholinesteraseinhibitory activities.Especially compound 5a; 3-[(5-(phenylamino)-1,3,4-thiadiazol-2-yl]methyl-2-(thiophen-3-ylmethyl)quinazolin-4(3H)-onehas the best urease inhibition result with 13.30 ± 0.15 µg/mL IC50 value, and it also has the best acetylcholinesterase inhibition with 20.30 ± 0.15 µg/mL IC50 value.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
4.40
自引率
8.30%
发文量
230
审稿时长
5.6 months
期刊介绍:
JICS is an international journal covering general fields of chemistry. JICS welcomes high quality original papers in English dealing with experimental, theoretical and applied research related to all branches of chemistry. These include the fields of analytical, inorganic, organic and physical chemistry as well as the chemical biology area. Review articles discussing specific areas of chemistry of current chemical or biological importance are also published. JICS ensures visibility of your research results to a worldwide audience in science. You are kindly invited to submit your manuscript to the Editor-in-Chief or Regional Editor. All contributions in the form of original papers or short communications will be peer reviewed and published free of charge after acceptance.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


