原位形成生物分子凝聚物,作为细胞内药物库,用于增强化疗效果
IF 26.8
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
摘要
由细胞内液-液相分离产生的生物分子凝聚物可为富集和延长小分子药物在细胞内的保留时间提供一种方法。在这里,我们报告了一种原位受控形成生物分子凝聚体的方法,这种凝聚体是富集和保留癌细胞中化疗药物的贮库,并表明这种方法可用于提高抗药性肿瘤小鼠的抗肿瘤疗效。该方法通过反式环辛烯和四嗪基团之间的点击释放反应,将组蛋白作为带正电荷的蛋白质与多柔比星插入的 DNA 链生物正交连接起来。这种反应暂时阻碍了组蛋白在体外的相分离,有利于启动细胞内的液-液相分离,并导致生物分子凝聚物的形成,这种凝聚物足够大,可以保留在肿瘤细胞内。在细胞内可控地形成生物分子凝聚物作为药物储存库,可为提高癌症疗法的疗效提供新的选择。本文章由计算机程序翻译,如有差异,请以英文原文为准。
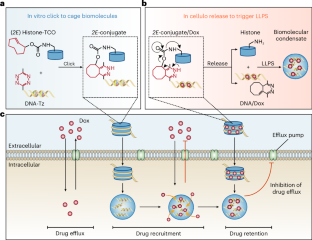
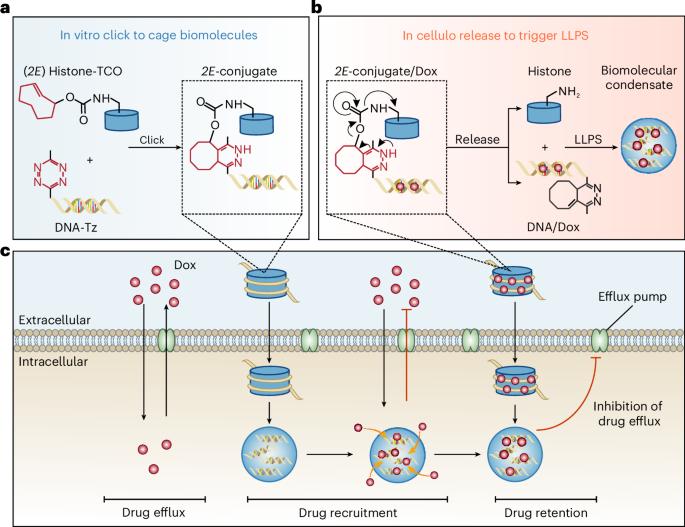
In situ formation of biomolecular condensates as intracellular drug reservoirs for augmenting chemotherapy
Biomolecular condensates, which arise from liquid–liquid phase separation within cells, may provide a means of enriching and prolonging the retention of small-molecule drugs within cells. Here we report a method for the controlled in situ formation of biomolecular condensates as reservoirs for the enrichment and retention of chemotherapeutics in cancer cells, and show that the approach can be leveraged to enhance antitumour efficacies in mice with drug-resistant tumours. The method involves histones as positively charged proteins and doxorubicin-intercalated DNA strands bioorthogonally linked via a click-to-release reaction between trans-cyclooctene and tetrazine groups. The reaction temporarily impaired the phase separation of histones in vitro, favoured the initiation of liquid–liquid phase separation within cells and led to the formation of biomolecular condensates that were sufficiently large to be retained within tumour cells. The controlled formation of biomolecular condensates as drug reservoirs within cells may offer new options for boosting the efficacies of cancer therapies. The controlled in situ formation of biomolecular condensates as intracellular reservoirs for the enrichment and retention of a chemotherapeutic in cancer cells enhanced antitumour activities in mice with drug-resistant tumours.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Biomedical Engineering
Medicine-Medicine (miscellaneous)
CiteScore
45.30
自引率
1.10%
发文量
138
期刊介绍:
Nature Biomedical Engineering is an online-only monthly journal that was launched in January 2017. It aims to publish original research, reviews, and commentary focusing on applied biomedicine and health technology. The journal targets a diverse audience, including life scientists who are involved in developing experimental or computational systems and methods to enhance our understanding of human physiology. It also covers biomedical researchers and engineers who are engaged in designing or optimizing therapies, assays, devices, or procedures for diagnosing or treating diseases. Additionally, clinicians, who make use of research outputs to evaluate patient health or administer therapy in various clinical settings and healthcare contexts, are also part of the target audience.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


