通过β-酮唑和芳基吖嗪的有机催化环加成生成唑代三唑
IF 1.9
4区 化学
Q3 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
报告了一种在室温下通过有机催化 β-酮唑和芳基叠氮化物之间的 [3+2] 环加成法合成新型唑取代三唑的高效无金属区域选择性方法。在常温反应条件下,无需使用柱层析作为纯化技术,所提出的方法对 β-酮唑和芳基叠氮化物中的各种取代基都有很好的耐受性,并且具有良好到极佳的收率(81-94 %)。本文章由计算机程序翻译,如有差异,请以英文原文为准。
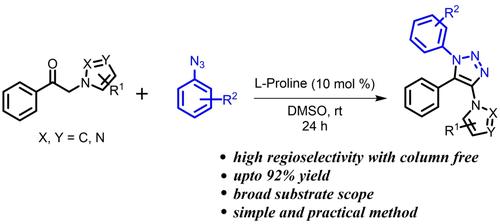
Azole-Substituted Triazole Formation through Organocatalytic Cycloaddition of β-keto Azoles and Aryl Azides
A highly efficient metal free regioselective approach for the synthesis of a novel azole substituted triazoles via organocatalytic [3+2] cycloaddition between β-keto azoles and aryl azides at room temperature is reported. The proposed methodology is well tolerated with range of substituents in the β-keto azoles and aryl azides with good to excellent yield (81–94 %) without column chromatography as purification technique under ambient reaction conditions.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ChemistrySelect
Chemistry-General Chemistry
CiteScore
3.30
自引率
4.80%
发文量
1809
审稿时长
1.6 months
期刊介绍:
ChemistrySelect is the latest journal from ChemPubSoc Europe and Wiley-VCH. It offers researchers a quality society-owned journal in which to publish their work in all areas of chemistry. Manuscripts are evaluated by active researchers to ensure they add meaningfully to the scientific literature, and those accepted are processed quickly to ensure rapid online publication.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


