通过延长π桥和意外的分子间去质子化实现苯酚-π-丙二腈衍生物的近红外荧光
IF 4.1
3区 工程技术
Q2 CHEMISTRY, APPLIED
引用次数: 0
摘要
苯酚-π-丙二腈衍生物是通过维蒂希反应延长乙烯π桥并在克诺文纳格尔缩合之后得到的近红外荧光苯酚-π-丙二腈衍生物。除了常规的 D-A 效应外,苯基环上的一个电子吸附基团引发的苯酚基团的意外分子间去质子化作用将发射波长推至 762 纳米,并产生了高达 323 纳米的大斯托克斯偏移。π桥的延伸和苯酚基团意外的分子间去质子化为构建低分子量近红外荧光团提供了一种可行的策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
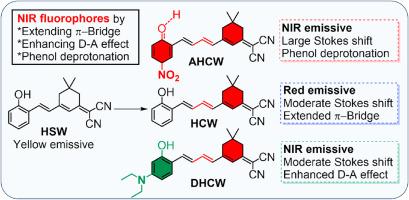
NIR fluorescence of phenol-π-malononitrile derivatives achieved by extending π-bridge and unexpected intermolecular deprotonation
NIR fluorescent phenol-π-malononitrile derivatives were achieved by extending vinylene π-bridge through Wittig reaction and following Knoevenagel condensation. In addition to regular D-A effect, unexpected intermolecular deprotonation of phenol group triggered by an electron-withdrawing group on phenyl ring pushed emissions to 762 nm with a large Stokes shift up to 323 nm. The extending of π-bridge and unexpected intermolecular deprotonation of phenol group promised a feasible strategy for construction of low-molecular weight NIR fluorophores.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Dyes and Pigments
工程技术-材料科学:纺织
CiteScore
8.20
自引率
13.30%
发文量
933
审稿时长
33 days
期刊介绍:
Dyes and Pigments covers the scientific and technical aspects of the chemistry and physics of dyes, pigments and their intermediates. Emphasis is placed on the properties of the colouring matters themselves rather than on their applications or the system in which they may be applied.
Thus the journal accepts research and review papers on the synthesis of dyes, pigments and intermediates, their physical or chemical properties, e.g. spectroscopic, surface, solution or solid state characteristics, the physical aspects of their preparation, e.g. precipitation, nucleation and growth, crystal formation, liquid crystalline characteristics, their photochemical, ecological or biological properties and the relationship between colour and chemical constitution. However, papers are considered which deal with the more fundamental aspects of colourant application and of the interactions of colourants with substrates or media.
The journal will interest a wide variety of workers in a range of disciplines whose work involves dyes, pigments and their intermediates, and provides a platform for investigators with common interests but diverse fields of activity such as cosmetics, reprographics, dye and pigment synthesis, medical research, polymers, etc.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


