铜催化的化学选择性烯烃交叉亲核烷基芳香化反应
IF 11.3
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
我们报告了一种通用的交叉核亲和烯烃烷基芳基化反应,将两种不同的硼酸(烷基和芳基)添加到乙烯基芳烃中,得到 1,1-二芳基烷烃。高化学选择性源于两种硼酸不同的反应活性:烷基硼酸被选择性地氧化成烷基自由基,而芳基硼酸则有利于与 Cu(II) 催化剂发生反金属反应。机理研究表明,原位生成的硼氧和添加的胺之间的路易斯酸-路易斯碱相互作用对选择性至关重要。本研究提供了 37 个例子,涉及结构和电子多样性的烷基、乙烯基和芳基偶联剂。在制备匹莫齐特和抗癌剂类似物时,证明了这种合成方法的实用性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
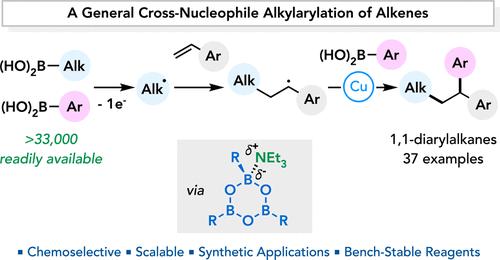
Chemoselective Cu-Catalyzed Cross-Nucleophile Alkylarylation of Alkenes
We report a general cross-nucleophile alkene alkylarylation that adds two different boronic acids, an alkyl and an aryl, across a vinylarene to afford 1,1-diarylalkanes. The high chemoselectivity originates from the distinct reactivities of the two boronic acids: the alkylboronic acid is selectively oxidized to an alkyl radical, while the arylboronic acid favors transmetalation with the Cu(II) catalyst. Mechanistic studies suggest that Lewis acid–Lewis base interactions between in situ generated boroxines and added amine are critical for selectivity. A scope of 37 examples is presented with structurally and electronically diverse alkyl, vinyl, and aryl coupling partners. The synthetic utility is demonstrated in the preparation of pimozide and anticancer agent analogues.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


