氨诱导的溶酶体和线粒体损伤导致效应 CD8+ T 细胞死亡
IF 19.1
1区 生物学
Q1 CELL BIOLOGY
引用次数: 0
摘要
氨被认为是一种细胞毒素,血液中氨的增加会损害细胞功能。然而,这种毒素是否以及如何在病理生理条件下引发细胞死亡仍不清楚。在这里,我们发现氨在效应 T 细胞中诱导了一种不同形式的细胞死亡。我们发现,快速增殖的 T 细胞利用谷氨酰胺溶解作用在线粒体中释放氨,然后将氨转运到溶酶体并储存起来。过量的氨积累会增加溶酶体的 pH 值,导致溶酶体氨储存终止和氨反流进入线粒体,从而导致线粒体损伤和细胞死亡,其特征是溶酶体碱化、线粒体肿胀和自噬通量受损。抑制谷氨酰胺溶解或阻断溶酶体碱化可防止氨诱导的 T 细胞死亡,并改善基于 T 细胞的抗肿瘤免疫疗法。这些发现确定了一种不同于以往已知机制的独特细胞死亡形式。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Ammonia-induced lysosomal and mitochondrial damage causes cell death of effector CD8+ T cells
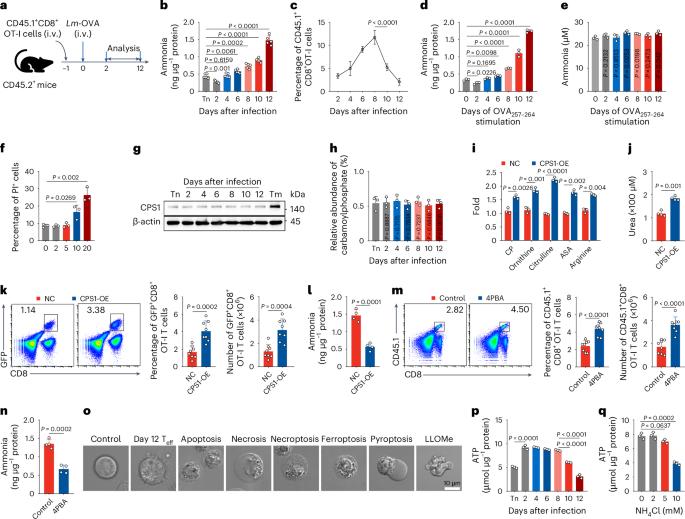
Ammonia is thought to be a cytotoxin and its increase in the blood impairs cell function. However, whether and how this toxin triggers cell death under pathophysiological conditions remains unclear. Here we show that ammonia induces a distinct form of cell death in effector T cells. We found that rapidly proliferating T cells use glutaminolysis to release ammonia in the mitochondria, which is then translocated to and stored in the lysosomes. Excessive ammonia accumulation increases lysosomal pH and results in the termination of lysosomal ammonia storage and ammonia reflux into mitochondria, leading to mitochondrial damage and cell death, which is characterized by lysosomal alkalization, mitochondrial swelling and impaired autophagic flux. Inhibition of glutaminolysis or blocking lysosomal alkalization prevents ammonia-induced T cell death and improves T cell-based antitumour immunotherapy. These findings identify a distinct form of cell death that differs from previously known mechanisms. Zhang, Liu and colleagues identify and characterize cell death in rapidly proliferating CD8+ T cells resulting from excessive ammonia accumulation and subsequent lysosomal dysfunction and mitochondrial damage.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Cell Biology
生物-细胞生物学
CiteScore
28.40
自引率
0.90%
发文量
219
审稿时长
3 months
期刊介绍:
Nature Cell Biology, a prestigious journal, upholds a commitment to publishing papers of the highest quality across all areas of cell biology, with a particular focus on elucidating mechanisms underlying fundamental cell biological processes. The journal's broad scope encompasses various areas of interest, including but not limited to:
-Autophagy
-Cancer biology
-Cell adhesion and migration
-Cell cycle and growth
-Cell death
-Chromatin and epigenetics
-Cytoskeletal dynamics
-Developmental biology
-DNA replication and repair
-Mechanisms of human disease
-Mechanobiology
-Membrane traffic and dynamics
-Metabolism
-Nuclear organization and dynamics
-Organelle biology
-Proteolysis and quality control
-RNA biology
-Signal transduction
-Stem cell biology
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


