HIV-1 广泛中和抗体三联疗法的安全性和抗病毒效果:1/2a 期试验
IF 58.7
1区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
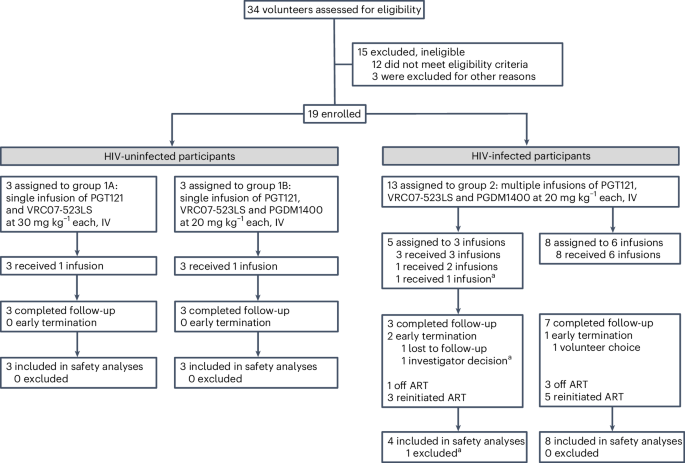
迄今为止,人类免疫缺陷病毒 1 型(HIV-1)特异性广谱中和单克隆抗体(bNAbs)在作为单药或两种抗体的鸡尾酒疗法1,2,3,4 时已显示出短暂的病毒抑制作用。三种 bNAbs 联合使用可提高对全球病毒的中和覆盖率,从而更有效地抑制病毒逃逸和反弹5,6,7。在此,我们进行了一项开放标签、分两部分的研究,第一部分评估了在 6 名未感染 HIV 的成人中单次静脉注射 PGT121、PGDM1400 和 VRC07-523LS 的效果,第二部分评估了在 12 名中断抗逆转录病毒疗法(ART)的 HIV 感染者中每月最多输注 6 次这三种 bNAbs 的多中心试验效果。第一部分的主要终点是安全性、耐受性和药代动力学,第二部分的次要终点是抗逆转录病毒疗法中断后的抗病毒活性、CD4+ T细胞计数的变化以及与bNAb耐药性相关的HIV-1序列突变的发生。试验达到了预设终点。bNAb治疗总体上安全且耐受性良好。在第二部分中,83%的参与者(12 人中有 10 人)在抗体治疗期间至少维持了 28 周的病毒学抑制,42%的参与者(12 人中有 5 人)至少维持了 38-44 周的病毒学抑制,尽管血清 bNAb 浓度已降至较低或检测不到的水平。在探索性分析中,两名患者的早期病毒反弹与对 PGT121 和 PGDM1400 的基线耐药有关,而五名患者的长期病毒控制与 bNAb 治疗后免疫激活、T 细胞衰竭和促炎信号转导的减少有关。我们的数据表明,在没有抗逆转录病毒疗法的情况下,三联 bNAb 鸡尾酒具有抑制 HIV-1 的潜力。ClinicalTrials.gov 注册:NCT03721510。本文章由计算机程序翻译,如有差异,请以英文原文为准。

Safety and antiviral effect of a triple combination of HIV-1 broadly neutralizing antibodies: a phase 1/2a trial
Human immunodeficiency virus type 1 (HIV-1)-specific broadly neutralizing monoclonal antibodies (bNAbs) have to date shown transient viral suppression when administered as monotherapy or as a cocktail of two antibodies1–4. A combination of three bNAbs provides improved neutralization coverage of global viruses, which may more potently suppress viral escape and rebound5–7. Here we performed an open-label, two-part study evaluating a single intravenous dose of HIV-1 bNAbs, PGT121, PGDM1400 and VRC07-523LS, in six adults without HIV in part 1 and a multicenter trial of up to six monthly infusions of these three bNAbs in 12 people living with HIV with an antiretroviral therapy (ART) interruption in part 2. The primary endpoints were safety, tolerability and pharmacokinetics, and the secondary endpoints in part 2 were antiviral activity following ART discontinuation, changes in CD4+ T cell counts and development of HIV-1 sequence mutations associated with bNAb resistance. The trial met its prespecified endpoints. The bNAb treatment was generally safe and well tolerated. In part 2, 83% of participants (10 of 12) maintained virologic suppression for the duration of antibody therapy for at least 28 weeks, and 42% of participants (5 of 12) showed virologic suppression for at least 38–44 weeks, despite the decline of serum bNAb concentrations to low or undetectable levels. In exploratory analyses, early viral rebound in two individuals correlated with baseline resistance to PGT121 and PGDM1400, whereas long-term virologic control in five individuals correlated with reduced immune activation, T cell exhaustion and proinflammatory signaling following bNAb therapy. Our data show the potential of a triple bNAb cocktail to suppress HIV-1 in the absence of ART. ClinicalTrials.gov registration: NCT03721510 . In a first-in-human trial of a triple combination of broadly neutralizing antibodies in people living with HIV, 83% of participants maintained virologic suppression for the duration of antibody therapy, with 42% showing virologic suppression for at least 38–44 weeks, despite the decline of serum antibody concentrations to low or undetectable levels.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Medicine
医学-生化与分子生物学
CiteScore
100.90
自引率
0.70%
发文量
525
审稿时长
1 months
期刊介绍:
Nature Medicine is a monthly journal publishing original peer-reviewed research in all areas of medicine. The publication focuses on originality, timeliness, interdisciplinary interest, and the impact on improving human health. In addition to research articles, Nature Medicine also publishes commissioned content such as News, Reviews, and Perspectives. This content aims to provide context for the latest advances in translational and clinical research, reaching a wide audience of M.D. and Ph.D. readers. All editorial decisions for the journal are made by a team of full-time professional editors.
Nature Medicine consider all types of clinical research, including:
-Case-reports and small case series
-Clinical trials, whether phase 1, 2, 3 or 4
-Observational studies
-Meta-analyses
-Biomarker studies
-Public and global health studies
Nature Medicine is also committed to facilitating communication between translational and clinical researchers. As such, we consider “hybrid” studies with preclinical and translational findings reported alongside data from clinical studies.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


