与烷基羧酸的γ-乳酸化有关的铁(V)-氧代羰基分子内 C-H 氧化作用
IF 11.3
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
在各种铁依赖性加氧酶中,高价氧铁物种被用作氧化剂。从大自然中汲取灵感,人们开发了一些非血红素铁复合物作为催化剂,通过假定的氧铁(V)物种(类似于为里斯克加氧酶提出的那些物种)的介导,引起 C-H 氧化。事实证明,在这些铁催化的 C-H 氧化反应中加入羧酸非常有利于提高产物产量和选择性,这表明铁(V)-氧代羧基物种的直接参与。当羧酸官能团存在于烷烃底物中时,它可作为一个定向基团,促成选择性分子内 γ-C-H 羟基化反应,最终生成 γ-内酯。虽然这一机理框架得到了以往机理研究的有力支持,但对关键的铁(V)-氧代羧基中间体及其参与导致内酯化的选择性 γ-C-H 氧化作用的能力的直接光谱检测尚未完成。在这项工作中,我们生成了一系列定义明确的铁(V)-氧代羧基物种(2c-2f),它们在结合的羧酸配体性质上有所不同。2c-2f 物种通过一系列光谱技术进行表征,包括紫外-可见光谱、冷喷雾电离质谱(CSI-MS),以及在某些情况下的 EPR 和莫斯鲍尔光谱。我们证明,2c-2f 以立体定向的方式对羧酸配体进行了位点选择性的 γ-内酯化反应,从而明确地确定了金属氧羧基物种是催化羧酸 γ-内酯化反应中强大而又具有选择性的 C-H 裂解物种。反应性实验证实,γ-内酯的分子内形成与外部烷烃和烯烃的分子间氧化是相互竞争的。最后,机理研究和 DFT 计算都支持这样一种机理,即羧酸配体的 γ 位上的 C-H 被氧化分子选择性地裂解,然后羧酸快速反弹,最终选择性地形成 γ-内酯。本文章由计算机程序翻译,如有差异,请以英文原文为准。
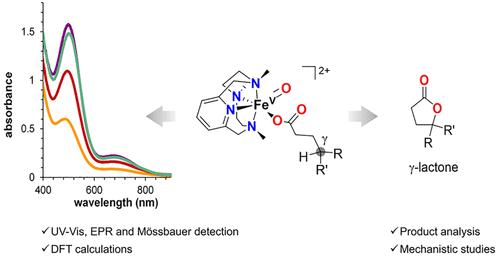
Intramolecular C–H Oxidation in Iron(V)-oxo-carboxylato Species Relevant in the γ-Lactonization of Alkyl Carboxylic Acids
High-valent oxoiron species have been invoked as oxidizing agents in a variety of iron-dependent oxygenases. Taking inspiration from nature, selected nonheme iron complexes have been developed as catalysts to elicit C–H oxidation through the mediation of putative oxoiron(V) species, akin to those proposed for Rieske oxygenases. The addition of carboxylic acids in these iron-catalyzed C–H oxidations has proved highly beneficial in terms of product yields and selectivities, suggesting the direct involvement of iron(V)-oxo-carboxylato species. When the carboxylic acid functionality is present in the alkane substrate, it acts as a directing group, enabling the selective intramolecular γ-C–H hydroxylation that eventually affords γ-lactones. While this mechanistic frame is solidly supported by previous mechanistic studies, direct spectroscopic detection of the key iron(V)-oxo-carboxylato intermediate and its competence for engaging in the selective γ–C–H oxidation leading to lactonization have not been accomplished. In this work, we generate a series of well-defined iron(V)-oxo-carboxylato species (2c–2f) differing in the nature of the bound carboxylate ligand. Species 2c–2f are characterized by a set of spectroscopic techniques, including UV–vis spectroscopy, cold-spray ionization mass spectrometry (CSI-MS), and, in selected cases, EPR and Mössbauer spectroscopies. We demonstrate that 2c–2f undergo site-selective γ-lactonization of the carboxylate ligand in a stereoretentive manner, thus unequivocally identifying metal-oxo-carboxylato species as the powerful yet selective C–H cleaving species in catalytic γ-lactonization reactions of carboxylic acids. Reactivity experiments confirm that the intramolecular formation of γ-lactones is in competition with the intermolecular oxidation of external alkanes and olefins. Finally, mechanistic studies, together with DFT calculations, support a mechanism involving a site-selective C–H cleavage in the γ-position of the carboxylate ligand by the oxo moiety, followed by a fast carboxylate rebound, eventually leading to the selective formation of γ-lactones.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


