二氧化碳电还原为乙烯和乙醇的关键中间体和铜活性位点
IF 60.1
1区 材料科学
Q1 ENERGY & FUELS
引用次数: 0
摘要
将二氧化碳(CO2RR)电化学还原为多碳产品,是将间歇性可再生能源电力储存为高附加值化学品并封闭碳循环的一项前景广阔的技术。其工业可扩展性要求电催化剂对某些产品(如乙烯或乙醇)具有高度选择性。然而,由于决定催化剂选择性的特性仍然难以捉摸,因此在设计定制材料方面存在巨大的知识差距。在此,我们结合铜电催化剂的原位表面增强拉曼光谱和密度泛函理论,揭示了 CO2RR 到 C2+ 产物的反应方案。当*OC-CO(H)二聚体在配位不足的 Cu 位点上通过 CO 偶联形成时,乙烯就生成了。乙醇路线只有在具有深 s 带状态的高度压缩和畸变 Cu 域存在的情况下,才会通过关键的中间体 *OCHCH2 出现。通过识别和跟踪关键的中间产物和特定的活性位点,我们的工作为在合理设计的催化剂上有选择地脱钩乙烯和乙醇的生产提供了指导。本文章由计算机程序翻译,如有差异,请以英文原文为准。
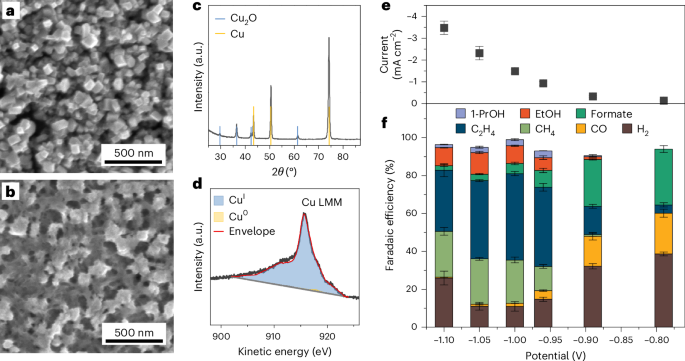

Key intermediates and Cu active sites for CO2 electroreduction to ethylene and ethanol
Electrochemical reduction of CO2 (CO2RR) to multi-carbon products is a promising technology to store intermittent renewable electricity into high-added-value chemicals and close the carbon cycle. Its industrial scalability requires electrocatalysts to be highly selective to certain products, such as ethylene or ethanol. However, a substantial knowledge gap prevents the design of tailor-made materials, as the properties ruling the catalyst selectivity remain elusive. Here we combined in situ surface-enhanced Raman spectroscopy and density functional theory on Cu electrocatalysts to unveil the reaction scheme for CO2RR to C2+ products. Ethylene generation occurs when *OC–CO(H) dimers form via CO coupling on undercoordinated Cu sites. The ethanol route opens up only in the presence of highly compressed and distorted Cu domains with deep s-band states via the crucial intermediate *OCHCH2. By identifying and tracking the critical intermediates and specific active sites, our work provides guidelines to selectively decouple ethylene and ethanol production on rationally designed catalysts. Electrochemical reduction of CO2 can yield many different products; a better understanding of the key mechanisms at play is needed to guide the design of selective catalysts. Here the authors use in situ surface-enhanced Raman spectroscopy and simulations to elucidate reaction schemes for CO2 reduction to ethylene and ethanol.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Energy
Energy-Energy Engineering and Power Technology
CiteScore
75.10
自引率
1.10%
发文量
193
期刊介绍:
Nature Energy is a monthly, online-only journal committed to showcasing the most impactful research on energy, covering everything from its generation and distribution to the societal implications of energy technologies and policies.
With a focus on exploring all facets of the ongoing energy discourse, Nature Energy delves into topics such as energy generation, storage, distribution, management, and the societal impacts of energy technologies and policies. Emphasizing studies that push the boundaries of knowledge and contribute to the development of next-generation solutions, the journal serves as a platform for the exchange of ideas among stakeholders at the forefront of the energy sector.
Maintaining the hallmark standards of the Nature brand, Nature Energy boasts a dedicated team of professional editors, a rigorous peer-review process, meticulous copy-editing and production, rapid publication times, and editorial independence.
In addition to original research articles, Nature Energy also publishes a range of content types, including Comments, Perspectives, Reviews, News & Views, Features, and Correspondence, covering a diverse array of disciplines relevant to the field of energy.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


