通过有机光氧化过程实现镍催化的醇与芳基氯化物的脱氧交叉偶联
IF 11.3
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
利用天然丰富的材料进行亲电体交叉偶联在有机合成中具有重要价值。在此,我们以二芳基酮为光催化剂,通过光氧催化和镍催化的融合,建立了一种以醇和工业首选芳基氯化物为偶联剂的高效脱氧交叉偶联反应。该方法具有底物范围广、官能团耐受性高的特点,可直接用于结构复杂的天然产品和药物(包括 C-4 烷基化吡啶)的放大合成和后期改性。该方法很可能是通过 HAT 和 β 裂解过程,从苯并恶唑盐-醇加合物中形成烷基自由基。本文章由计算机程序翻译,如有差异,请以英文原文为准。
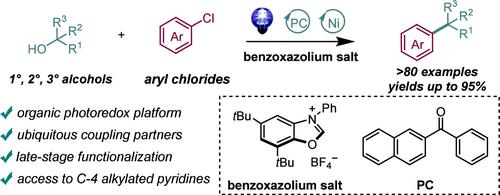
Ni-Catalyzed Deoxygenative Cross-Coupling of Alcohols with Aryl Chlorides via an Organic Photoredox Process
Cross-electrophile coupling from naturally abundant materials is of significant value in organic synthesis. Herein, we established a highly efficient deoxygenative cross-coupling reaction using alcohols and industrial preferred aryl chlorides as coupling partners by the merging of photoredox and nickel catalysis with diaryl ketone as a photocatalyst. This methodology features a broad substrate scope and high functional group tolerance, with straightforward application of scale-up synthesis and late-stage modification of structurally complex natural products and pharmaceuticals, including C-4 alkylated pyridines. This protocol most likely proceeds via a HAT and β-scission process to form alkyl radicals from benzoxazolium salt-alcohol adducts.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


